Hyper-Cross-linked Cellulose Nanofibrils with Spontaneous and Reversible Adsorption of Aromatic Pollutants from Water as a Valid Alternative to Fossil-Based Adsorbents
- PMID: 40540321
- PMCID: PMC12232286
- DOI: 10.1021/acsami.5c05009
Hyper-Cross-linked Cellulose Nanofibrils with Spontaneous and Reversible Adsorption of Aromatic Pollutants from Water as a Valid Alternative to Fossil-Based Adsorbents
Abstract
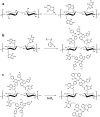
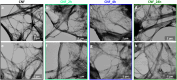
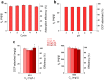
In this work, a novel high surface area adsorbent based on cellulose and inspired by hyper-cross-linked polymers was designed. Cellulose nanofibrils (CNF) were functionalized with poly(vinylbenzyl chloride) and hyper-cross-linked through Friedel-Crafts alkylation, yielding a micro/mesoporous material characterized by a specific surface area of 409 m2/g, microporous fraction of 50%, and biobased content of about 70 wt %. The functionalized CNF, tested for the adsorption of 2,4-dichlorophenol (DCP) from water at 298 K, were able to remove 90% of the pollutant from a 62.5 mg/L DCP solution and adsorb 284 mg/g at a higher concentration (1000 mg/L). Thermodynamic studies demonstrated the multilayer adsorption of the hyper-cross-linked CNF, the exothermic nature of the process, and its spontaneity. The hyper-cross-linked cellulose nanofibrils were reusable with efficiency above 98% in 5 subsequent cycles. The adsorption performance was stable across varying pH levels, and interference from natural organic matter (e.g., humic acids) was minimal (<10%). This work marked a promising step toward more sustainable sorbent materials by demonstrating the potential of cellulose nanofibrils as functional scaffolds. The strategy could be extended to waste-derived cellulose sources and biobased aromatic compounds, paving the way for fully renewable porous adsorbents.
Keywords: adsorption; aromatic pollutants; cellulose; hyper-cross-linking; regeneration.
Figures








Similar articles
-
Removal of fluoride from aqueous solution using high surface area activated carbon derived from fish scale solid waste.Environ Sci Pollut Res Int. 2025 Jun;32(26):15929-15941. doi: 10.1007/s11356-025-36638-3. Epub 2025 Jun 20. Environ Sci Pollut Res Int. 2025. PMID: 40542193
-
Integration of magnetic graphene oxide and office waste paper-derived fibrillated cellulose into a composite adsorbent for effective tetracycline elimination.RSC Adv. 2025 Jun 30;15(27):22138-22153. doi: 10.1039/d5ra00877h. eCollection 2025 Jun 23. RSC Adv. 2025. PMID: 40589466 Free PMC article.
-
[Recent applications of porous-material-based adsorbents for extracting pesticide residues from environmental and foodstuff samples].Se Pu. 2025 Jul;43(7):713-725. doi: 10.3724/SP.J.1123.2024.12009. Se Pu. 2025. PMID: 40610766 Free PMC article. Review. Chinese.
-
From waste to water savior: Fe-Mn-boosted waste-derived cellulose for sustainable tetracycline treatment.Int J Biol Macromol. 2025 Sep 1;327(Pt 1):147286. doi: 10.1016/j.ijbiomac.2025.147286. Online ahead of print. Int J Biol Macromol. 2025. PMID: 40897277
-
Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease.Cochrane Database Syst Rev. 2001;(3):CD003234. doi: 10.1002/14651858.CD003234. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003234. doi: 10.1002/14651858.CD003234.pub2. PMID: 11687058 Updated.
References
-
- Akhtar N., Syakir Ishak M. I., Bhawani S. A., Umar K.. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water. 2021;13(19):2660. doi: 10.3390/w13192660. - DOI
-
- Bi J., Huang X., Wang J., Wang T., Wu H., Yang J., Lu H., Hao H.. Oil-Phase Cyclic Magnetic Adsorption to Synthesize Fe3O4@C@TiO2-Nanotube Composites for Simultaneous Removal of Pb(II) and Rhodamine B. Chem. Eng. J. 2019;366:50–61. doi: 10.1016/j.cej.2019.02.017. - DOI
-
- Chaudhry F. N., Malik M. F.. Factors Affecting Water Pollution: A Review. J. Ecosyst. Ecography. 2017;7(1):1000225. doi: 10.4172/2157-7625.1000225. - DOI
-
- Makinde Z. O., Louzada M., Mashazi P., Nyokong T., Khene S.. Electrocatalytic Behaviour of Surface Confined Pentanethio Cobalt (II) Binuclear Phthalocyanines towards the Oxidation of 4-Chlorophenol. Appl. Surf. Sci. 2017;425:702–712. doi: 10.1016/j.apsusc.2017.06.271. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

