Neuraminidase 1 secondary deficiency contributes to CNS pathology in neurological mucopolysaccharidoses via brain protein hypersialylation
- PMID: 40540388
- PMCID: PMC12352893
- DOI: 10.1172/JCI177430
Neuraminidase 1 secondary deficiency contributes to CNS pathology in neurological mucopolysaccharidoses via brain protein hypersialylation
Abstract
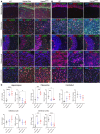
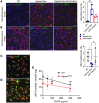
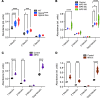
Mucopolysaccharidoses (MPS) are lysosomal storage diseases caused by defects in catabolism of glycosaminoglycans. MPS I, II, III, and VII, which are associated with lysosomal accumulation of heparan sulphate (HS), manifest with neurological deterioration and currently lack effective treatments. We report that neuraminidase 1 (NEU1) activity is drastically reduced in brain tissues of patients with neurological MPS and mouse models but not in neurological lysosomal disorders without HS storage. Accumulated HS disrupts the lysosomal multienzyme complex of NEU1 with cathepsin A, β-galactosidase (GLB1), and glucosamine-6-sulfate sulfatase (GALNS), leading to NEU1 deficiency and partial GLB1 and GALNS deficiencies in cortical tissues and induced pluripotent stem cell-derived (iPSC-derived) cortical neurons of patients with neurological MPS. Increased sialylation of N-linked glycans in brains of patients with MPS and mice implicated insufficient processing of sialylated glycans, except for polysialic acid. Correction of NEU1 activity in MPS IIIC mice by lentiviral (LV) gene transfer ameliorated previously identified hallmarks of the disease, including memory impairment, behavioral traits, and reduced levels of excitatory synapse markers VGLUT1 and PSD95. Overexpression of NEU1 also restored levels of VGLUT1/PSD95-positive puncta in cortical iPSC-derived MPS IIIA neurons. Our results demonstrate that HS-induced secondary NEU1 deficiency and aberrant sialylation of brain glycoproteins constitute what we believe is a novel pathological pathway in the neurological MPS spectrum crucially contributing to CNS pathology.
Keywords: Genetic diseases; Genetics; Glycobiology; Lysosomes; Neuroscience.
Figures







Update of
-
Secondary deficiency of neuraminidase 1 contributes to CNS pathology in neurological mucopolysaccharidoses via hypersialylation of brain glycoproteins.bioRxiv [Preprint]. 2024 Apr 27:2024.04.26.587986. doi: 10.1101/2024.04.26.587986. bioRxiv. 2024. Update in: J Clin Invest. 2025 Jun 17;135(16):e177430. doi: 10.1172/JCI177430. PMID: 38712143 Free PMC article. Updated. Preprint.
Similar articles
-
Secondary deficiency of neuraminidase 1 contributes to CNS pathology in neurological mucopolysaccharidoses via hypersialylation of brain glycoproteins.bioRxiv [Preprint]. 2024 Apr 27:2024.04.26.587986. doi: 10.1101/2024.04.26.587986. bioRxiv. 2024. Update in: J Clin Invest. 2025 Jun 17;135(16):e177430. doi: 10.1172/JCI177430. PMID: 38712143 Free PMC article. Updated. Preprint.
-
Arylsulfatase K inactivation causes mucopolysaccharidosis due to deficient glucuronate desulfation of heparan and chondroitin sulfate.Biochem J. 2020 Sep 18;477(17):3433-3451. doi: 10.1042/BCJ20200546. Biochem J. 2020. PMID: 32856704 Free PMC article.
-
AAV-mediated gene therapy for sialidosis.Mol Ther. 2024 Jul 3;32(7):2094-2112. doi: 10.1016/j.ymthe.2024.05.029. Epub 2024 May 25. Mol Ther. 2024. PMID: 38796704 Free PMC article.
-
Humanistic burden of mucopolysaccharidoses: a systematic literature review.Curr Med Res Opin. 2024 Apr;40(4):709-722. doi: 10.1080/03007995.2024.2316213. Epub 2024 Mar 8. Curr Med Res Opin. 2024. PMID: 38328952
-
Current and potential therapeutic strategies for mucopolysaccharidoses.J Clin Pharm Ther. 2014 Jun;39(3):215-24. doi: 10.1111/jcpt.12136. Epub 2014 Feb 25. J Clin Pharm Ther. 2014. PMID: 24612142
Cited by
-
NEU1-Mediated Extracellular Vesicle Glycosylation in Alzheimer's Disease: Mechanistic Insights into Intercellular Communication and Therapeutic Targeting.Pharmaceuticals (Basel). 2025 Jun 19;18(6):921. doi: 10.3390/ph18060921. Pharmaceuticals (Basel). 2025. PMID: 40573316 Free PMC article. Review.
References
-
- Neufeld EF, Muenzer J. The Mucopolysaccharidoses. In Beaudet AL, eds. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw Hill; 2014.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

