Hepatitis B Virus Variants and Cytokine Patterns in Acute Liver Failure and Transplant-Free Survival
- PMID: 40540404
- PMCID: PMC12180610
- DOI: 10.1111/liv.70175
Hepatitis B Virus Variants and Cytokine Patterns in Acute Liver Failure and Transplant-Free Survival
Abstract
Background & aims: Only 25% of hepatitis B-related acute liver failure (HBV-ALF) patients survive without liver transplantation (transplant-free survival, TFS). There is limited study of immunological and virological profiles in these patients. We analysed the association between hepatitis B viremia and cytokine patterns on TFS of HBV-ALF patients.
Methods: We identified 48 acute and 20 history of HBV infection ALF patients from the US ALF Study Group registry (> 3400 patients). The inclusion criteria were age > 18 years, diagnosis of HBV-ALF with hepatic encephalopathy and INR ≥ 1.5. Data were collected from ICU admission through day 21. Serum collected at admission and at days 3-5 were used for cytokine quantification by Luminex and novel HBV biomarkers, genotypes and variants.
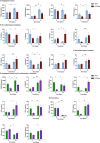
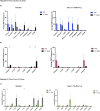
Results: In 48 acute (50% F, median age 40 years) and 20 history/reactivation (40% F, median age 53 years) HBV-ALF patients, there were 26 (54%) and 5 (25%) TFS, respectively. Detectable HBV DNA by clinical PCR assay (median 3.39 log10IU/mL, aOR 5.308; 95% CI: 1.217-23.155, p = 0.026) and qAHBc levels (median 4.5 log10IU/mL, aOR 4.466, 95% CI: 0.968-20.608, p = 0.050) were associated with TFS in acute HBV-ALF patients. HBV variants associated with anti-viral immune escape were more frequently detected in acute HBV-ALF TFS patients compared to non-TFS (p < 0.05). TFS with acute HBV-ALF had higher angiogenic factors (PDGF-AA, p = 0.008; PDGF-BB, p = 0.0006; VEGF-A, p = 0.014) and lower pro-inflammatory cytokine levels (IL-1α, p = 0.031; IL-2, p = 0.014; IL-6, p = 0.039). Significant differences in HBV viremia were not observed in history/reactivation of HBV-ALF patients.
Conclusions: Acute HBV-ALF patients with TFS were often viremic with immune escape variants, increased angiogenic factors and decreased pro-inflammatory cytokines.
Trial registration: ClinicalTrials.gov identifier: NCT00518440.
Keywords: acute liver failure; cytokines; hepatitis B; hepatitis B virus quasispecies; transplant‐free survival.
© 2025 The Author(s). Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
Nishi H. Patel, Annie Y. Chen, Carla Osiowy, Valerie Durkalski Mauldin, William M. Lee declare no conflicts of interest. Constantine J. Karvellas: Consulting ad hoc for Baxter, Grifols, Morphocell. Carla S. Coffin: Advisory boards and Speaker Fees: Altimmune Pharmaceuticals, Janssen, Roche, GSK (paid to the University of Calgary). Investigator Initiated Grants: Gilead, GSK, Janssen (paid to the University of Calgary the Canadian HBV Network).
Figures

References
-
- Khashab M., Tector A. J., and Kwo P. Y., “Epidemiology of Acute Liver Failure,” Current Gastroenterology Reports 9, no. 1 (2007): 66–73. - PubMed
-
- Dong V., Nanchal R., and Karvellas C. J., “Pathophysiology of Acute Liver Failure,” Nutrition in Clinical Practice 35, no. 1 (2020): 24–29. - PubMed
-
- Bernal W. and Wendon J., “Acute Liver Failure,” New England Journal of Medicine 369, no. 26 (2013): 2525–2534. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

