The adverse events of toripalimab in nasopharyngeal carcinoma based on FAERS database and bibliometric analysis
- PMID: 40540517
- PMCID: PMC12180722
- DOI: 10.1371/journal.pone.0326216
The adverse events of toripalimab in nasopharyngeal carcinoma based on FAERS database and bibliometric analysis
Abstract
Background: Toripalimab, a monoclonal antibody designed to target PD-1, has recently received approval from the U.S. Food and Drug Administration (FDA) for use as a first-line treatment in adults diagnosed with metastatic or recurrent locally advanced nasopharyngeal carcinoma (NPC). The purpose of this study is to utilize the FAERS database and bibliometric analysis to examine adverse events associated with toripalimab in real-world settings, thereby enhancing the safety management of clinical medications.
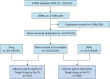
Methods: This research implemented a disproportionality analysis to assess the safety of toripalimab by reviewing all adverse event reports from the FAERS database dating back to 2004, wherein toripalimab was recognized as the main suspected medication. Various statistical techniques were applied in the analysis, such as the reporting odds ratio (ROR), proportional reporting ratio (PRR), multi-item gamma Poisson shrinker (MGPS), and Bayesian confidence propagation neural network (BCPNN), to evaluate the adverse events linked to toripalimab. CiteSpace is utilized to search for authors, countries, keywords, and various indicators within research fields, facilitating the identification of research hotspots and future trends.
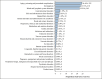
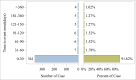
Results: From 2004 to 2024, 441 AEs linked to toripalimab were recorded across 27 SOCs. The top five SOCs were procedural complications, investigations, blood/lymphatic disorders, gastrointestinal disorders, and skin/subcutaneous disorders. At the PT level, the top five AEs by ROR were myelosuppression (n = 192, ROR 687.41), decreased granulocyte count (n = 11, ROR 515.72), immune-mediated hepatic disorder (n = 7, ROR 343.20), immune-mediated myocarditis (n = 3, ROR 214.68), and bicytopenia (n = 3, ROR 117.49). Additionally, 91.62% of AEs occurred within the first 30 days, and immune-related AEs were highlighted in bibliometric analysis.
Conclusion: This research provides initial safety information regarding the real-world application of toripalimab, affirming previously acknowledged adverse effects and concurrently uncovering new possible risks. These results could act as important cautionary evidence for healthcare professionals and pharmacists engaged in administering toripalimab for NPC.
Copyright: © 2025 Guo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Adverse drug reactions related to methotrexate: a real-world pharmacovigilance study using the FAERS database from 2004 to 2024.Front Immunol. 2025 Jun 4;16:1586361. doi: 10.3389/fimmu.2025.1586361. eCollection 2025. Front Immunol. 2025. PMID: 40534848 Free PMC article. Review.
-
Safety evaluation of irinotecan: a real-world disproportionality analysis using FAERS and JADER databases during the time period 2004-2024.Front Pharmacol. 2025 Jun 9;16:1516449. doi: 10.3389/fphar.2025.1516449. eCollection 2025. Front Pharmacol. 2025. PMID: 40552159 Free PMC article.
-
A pharmacovigilance study of vortioxetine based on data from the FDA adverse event reporting system.Sci Rep. 2025 Aug 7;15(1):28886. doi: 10.1038/s41598-025-13786-7. Sci Rep. 2025. PMID: 40775011 Free PMC article.
-
Unveiling potential adverse events associated with escitalopram oxalate: A real-world analysis based FDA adverse event reporting system database.J Psychopharmacol. 2024 Jun;38(6):567-578. doi: 10.1177/02698811241249651. Epub 2024 Apr 27. J Psychopharmacol. 2024. PMID: 38678377
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
References
-
- Chen Y, et al. The current advances and future directions of PD-1/PD-L1 blockade in head and neck squamous cell carcinoma (HNSCC) in the era of immunotherapy. Int Immunopharmacol. 2023;120:110329. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

