Humidity drives spontaneous OH oxidation of organic particles
- PMID: 40540571
- PMCID: PMC12180490
- DOI: 10.1126/sciadv.adx4507
Humidity drives spontaneous OH oxidation of organic particles
Abstract
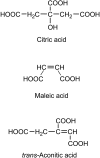
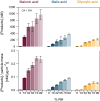
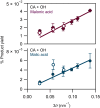
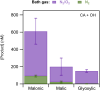
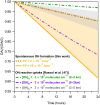
We report evidence that organic aerosols containing carboxylic acids can be spontaneously oxidized in the dark under normal atmospheric conditions due to interfacial hydroxyl radical production. Product formation is negligible under dry conditions and increases with increasing relative humidity. In a dioxygen-free environment, the oxidation efficiency is substantially decreased. Size-resolved measurements show an increase in the reactivity and product formation yields for smaller particles, correlated with their surface-to-volume ratio. Our findings suggest that spontaneous hydroxyl radical production at the air-water interface of organic nanodroplets may be an important pathway in their oxidation, especially during nighttime.
Figures
References
-
- J. H. Seinfeld, S. N. Pandis, Atmospheric Chemistry and Physics: From Air Pollution to Climate Change (John Wiley and Sons, 1998).
-
- Kanakidou M., Seinfeld J. H., Pandis S. N., Barnes I., Dentener F. J., Facchini M. C., Dingenen R. V., Ervens B., Nenes A., Nielsen C. J., Swietlicki E., Putaud J. P., Balkanski Y., Fuzzi S., Horth J., Moortgat G. K., Winterhalter R., Myhre C. E. L., Tsigaridis K., Vignati E., Stephanou E. G., Wilson J., Organic aerosol and global climate modelling: A review. Atmos. Chem. Phys. 5, 1053–1123 (2005).
-
- Pöschl U., Atmospheric aerosols: Composition, transformation, climate and health effects. Angew. Chem. Int. Ed. 44, 7520–7540 (2005). - PubMed
-
- Cappa C. D., Che D. L., Kessler S. H., Kroll J. H., Wilson K. R., Variations in organic aerosol optical and hygroscopic properties upon heterogeneous OH oxidation. J. Geophys. Res. 116, D15204 (2011).
LinkOut - more resources
Full Text Sources

