Metabolically driven flows enable exponential growth in macroscopic multicellular yeast
- PMID: 40540574
- PMCID: PMC12180493
- DOI: 10.1126/sciadv.adr6399
Metabolically driven flows enable exponential growth in macroscopic multicellular yeast
Abstract
The ecological and evolutionary success of multicellular lineages stems substantially from their increased size relative to unicellular ancestors. However, large size poses biophysical challenges, especially regarding nutrient transport: These constraints are typically overcome through multicellular innovations. Here, we show that an emergent biophysical mechanism-spontaneous fluid flows arising from metabolically generated density gradients-can alleviate constraints on nutrient transport, enabling exponential growth in nascent multicellular clusters of yeast lacking any multicellular adaptations for nutrient transport or fluid flow. Beyond a threshold size, the metabolic activity of experimentally evolved snowflake yeast clusters drives large-scale fluid flows that transport nutrients throughout the cluster at speeds comparable to those generated by ciliary actuation in extant multicellular organisms. These flows support exponential growth at macroscopic sizes that theory predicts should be diffusion limited. This demonstrates how simple physical mechanisms can act as a "biophysical scaffold" to support the evolution of multicellularity by opening up phenotypic possibilities before genetically encoded innovations.
Figures
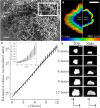
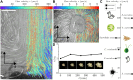

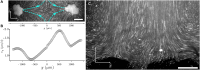
Update of
-
Metabolically-driven flows enable exponential growth in macroscopic multicellular yeast.bioRxiv [Preprint]. 2024 Jun 22:2024.06.19.599734. doi: 10.1101/2024.06.19.599734. bioRxiv. 2024. Update in: Sci Adv. 2025 Jun 20;11(25):eadr6399. doi: 10.1126/sciadv.adr6399. PMID: 38948761 Free PMC article. Updated. Preprint.
References
-
- Grosberg R. K., Strathmann R. R., The evolution of multicellularity: A minor major transition? Annu. Rev. Ecol. Evol. Syst. 38, 621–654 (2007).
-
- M. D. Herron, P. L. Conlin, W. C. Ratcliff, Eds., The Evolution of Multicellularity (CRC Press, 2022).
-
- Kai Tong G., Bozdag O., Ratcliff W. C., Selective drivers of simple multicellularity. Curr. Opin. Microbiol. 67, 102141 (2022). - PubMed
-
- Fisher S. A., Burggren W. W., Role of hypoxia in the evolution and development of the cardiovascular system. Antioxid. Redox Signal. 9, 1339–1352 (2007). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

