Molecular predictive biomarker testing in advanced thyroid cancer - a European consensus
- PMID: 40540622
- PMCID: PMC12242913
- DOI: 10.1530/ETJ-25-0024
Molecular predictive biomarker testing in advanced thyroid cancer - a European consensus
Abstract
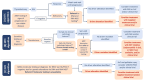
As new precision oncology therapies become available in the thyroid cancer (TC) treatment landscape, appropriate and timely biomarker testing is crucial for treatment selection and requires a multidisciplinary approach. Recently published European guidelines on advanced/metastatic TC management include a special focus on biomarker testing. However, to date, there remains a need for comprehensive European guidance for standardized molecular testing strategies in TC that encompass a broad set of targetable or potentially targetable alterations, timing of testing, and patients to be tested. This expert opinion article outlines consensus testing algorithms for differentiated TC, medullary TC, and anaplastic TC from a team of endocrinologists, oncologists, molecular biologists, and pathologists to provide standardized recommendations for physicians involved in treating patients with advanced TC. In the differentiated TC algorithm, patients recommended for comprehensive testing by DNA and RNA next-generation sequencing (NGS) include those whose disease has progressed on or is resistant to radioactive iodine treatment. The medullary TC algorithm recommends RET germline testing for all patients at diagnosis. For patients exhibiting high-risk clinical or pathological features and those whose disease progresses, somatic RET testing with NGS should be discussed and conducted before considering systemic treatment. As anaplastic TC is a highly aggressive disease, molecular reflex testing for BRAF mutations is recommended for all patients at diagnosis, followed by DNA and RNA NGS for those who test BRAF negative. The article also provides consensus recommendations on the use of tumor tissue for testing and on centralization of molecular testing involving multidisciplinary tumor boards.
Keywords: biomarkers; consensus; molecular testing; multidisciplinary; thyroid cancer.
Conflict of interest statement
AR has participated at advisory board meetings and/or as an invited speaker for Amgen, AstraZeneca, Bayer, BMS, Eli Lilly, Gilead, Merck, MSD, Pfizer, Roche, and Sanofi, and has received travel support from Gilead and Sanofi. JC has served as advisor and/or speaker for and/or received research support from Advanced Accelerator Applications, Advanz, Amgen, AstraZeneca, Bayer, Eisai, Eli Lilly, Esteve, Exelixis, Gilead, Hudchmed, Incyte, Ipsen, ITM, Merck Serono, Novartis, Pfizer, Roche, and Sanofi. MSD declares no competing interests. RE is a consultant for Bayer, Eisai, Eli Lilly, IPSEN, and Menarini. DF declares no competing interests. JH has served as advisor and/or speaker for and/or received funding for continuing medical education and/or research grants from AAA, Bayer, Eisai, Eli Lilly, HRA Pharma, IPSEN, ITM, Novartis, Pharma Mar, Roche, and Sanofi. BJ declares consultancy for AstraZeneca and employment by AstraZeneca, Bayer, Eisai, Exelixis, Novartis, OxiGene, Pfizer, and Roche for clinical trials, and has received payment for lecturing and developing educational presentations and/or received travel/meeting expenses from Ipsen, Novartis, and Sanofi. BJ is also a coauthor of patent application EP22460030, which is intended to grant an EPO patent. LDL has participated in advisory board meetings for and/or received conference honoraria from Bayer, Eisai, Eli Lilly, IPSEN, Istituto Gentili Srl, Janssen-Cilag, Merck Serono, MSD, New Bridge, Novartis, Roche, Sanofi, Seagen, and Sunpharma, and has received travel support from Gilead. KN has served on the speakers bureau for Eisai. GT declares no competing interests. SU declares no competing interests. LW has received honoraria for advisory roles from Bayer, Blueprint Medicines, Coherus, Eisai, Eli Lilly, Ellipses, EMD Serono, Exelixis, Illumina, Merck, Nested, Novartis, and Tubulis, and received honoraria for serving on a data safety monitoring board from PDS Biotechnology Corporation. RS, IMS, and PC are employees of Eli Lilly. LF has served as a consultant for Eisai, Eli Lilly, and Ipsen.
Figures
Similar articles
-
Characterization of the Thyroid Cancer Genomic Landscape by Plasma-Based Circulating Tumor DNA Next-Generation Sequencing.Thyroid. 2024 Feb;34(2):197-205. doi: 10.1089/thy.2023.0204. Epub 2023 Dec 22. Thyroid. 2024. PMID: 37962267
-
Multidisciplinary collaborative guidance on the assessment and treatment of patients with Long COVID: A compendium statement.PM R. 2025 Jun;17(6):684-708. doi: 10.1002/pmrj.13397. Epub 2025 Apr 22. PM R. 2025. PMID: 40261198
-
Next-Generation Sequencing-Based Testing Among Patients With Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer in the United States: Predictive Modeling Using Machine Learning Methods.JMIR Cancer. 2025 Jun 11;11:e64399. doi: 10.2196/64399. JMIR Cancer. 2025. PMID: 40497643 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
AGA Clinical Practice Update on GI Manifestations and Autonomic or Immune Dysfunction in Hypermobile Ehlers-Danlos Syndrome: Expert Review.Clin Gastroenterol Hepatol. 2025 Jul;23(8):1291-1302. doi: 10.1016/j.cgh.2025.02.015. Epub 2025 May 19. Clin Gastroenterol Hepatol. 2025. PMID: 40387691 Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

