Impact of Lean Body Mass-Based Oxaliplatin Dose Calculation on Neurotoxicity in Adjuvant Treatment of Stage III Colon Cancer: Results of the Phase II Randomized LEANOX Trial
- PMID: 40540704
- PMCID: PMC12316125
- DOI: 10.1200/JCO-24-02754
Impact of Lean Body Mass-Based Oxaliplatin Dose Calculation on Neurotoxicity in Adjuvant Treatment of Stage III Colon Cancer: Results of the Phase II Randomized LEANOX Trial
Erratum in
-
Erratum: Impact of Lean Body Mass-Based Oxaliplatin Dose Calculation on Neurotoxicity in Adjuvant Treatment of Stage III Colon Cancer: Results of the Phase II Randomized LEANOX Trial.J Clin Oncol. 2025 Dec 10;43(35):3774. doi: 10.1200/JCO-25-02239. Epub 2025 Oct 28. J Clin Oncol. 2025. PMID: 41150998 No abstract available.
Abstract
Purpose: Oxaliplatin-based adjuvant chemotherapy is used for stage III colon cancer, but may induce disabling neurotoxicity. We previously showed that the incidence of oxaliplatin-induced peripheral neurotoxicity (OIPN) is higher for oxaliplatin doses >3.09 mg per kg of lean body mass (LBM). This proof-of-concept, multicenter, randomized trial assessed whether LBM-based oxaliplatin dose adjustment reduces OIPN (ClinicalTrials.gov identifier: NCT03255434).
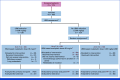
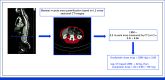
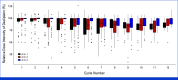
Methods: Among the patients with resected stage III colon cancer eligible for adjuvant leucovorin, fluorouracil, and oxaliplatin chemotherapy, those without LBM reduction received body surface area (BSA)-based oxaliplatin doses (85 mg/m2, arm 1). Patients with reduced LBM were randomly assigned (1:1) to receive BSA-based (arm 2) or LBM-based oxaliplatin doses (3.09 mg/kg LBM, arm 3). The primary end point was the percentage of patients without grade ≥2 OIPN in the first six cycles.
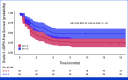
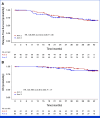
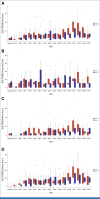
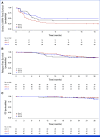
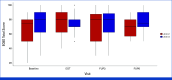
Results: In all, 33, 64, and 63 patients were enrolled in arms 1, 2, and 3, respectively (median age, 63 years; 52.5% of men; 89.3% Eastern Cooperative Oncology Group 0; 57.5% pT3; 60.6% pN1). The primary end point was achieved by 67.2% of patients in arm 3 versus 42.1% in arm 2 (P = .01). Longer grade ≥2 OIPN-free survival (hazard ratio [HR], 0.53 [95% CI, 0.34 to 0.84]; P = .01), longer time to grade ≥2 OIPN onset (P = .006), higher cumulative oxaliplatin doses without grade ≥2 OIPN (P = .044), and fewer oxaliplatin dose reductions (P < .001) were reported in arm 3. Relapse-free survival (HR, 1.05 [95% CI, 0.54 to 2.06]) and overall survival (OS; HR, 1.20 [95% CI, 0.36 to 3.92]) were similar in arms 2 and 3 (median follow-up of 38.6 months). Quality of Life Questionnaire Chemotherapy-Induced Peripheral Neuropathy 20 scores were better in arm 3.
Conclusion: In adjuvant settings for stage III colon cancer, using an LBM-based oxaliplatin dose significantly reduces OIPN and improves quality of life without affecting relapse-free survival and OS.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. - PubMed
-
- Grothey A. Oxaliplatin-safety profile: Neurotoxicity. Semin Oncol. 2003;30:5–13. - PubMed
-
- Pietrangeli A, Leandri M, Terzoli E, et al. Persistence of high-dose oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol. 2006;56:13–16. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

