Impact of age and sex on the efficacy and safety of ramucirumab plus paclitaxel as switch maintenance versus continuation of first-line oxaliplatin-based chemotherapy: a subgroup analysis of the ARMANI phase III trial
- PMID: 40540760
- PMCID: PMC12221353
- DOI: 10.1016/j.esmoop.2025.105329
Impact of age and sex on the efficacy and safety of ramucirumab plus paclitaxel as switch maintenance versus continuation of first-line oxaliplatin-based chemotherapy: a subgroup analysis of the ARMANI phase III trial
Abstract
Background: There is a growing interest in optimizing the therapeutic management of older cancer patients as well as understanding the sex-specific differences in terms of efficacy and safety of cancer treatments. However, limited data are available on the initial therapy of patients with advanced gastro-oesophageal cancer.
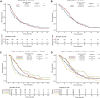
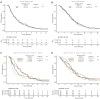
Materials and methods: The ARMANI phase III trial showed progression-free survival (PFS) and overall survival (OS) benefit with paclitaxel plus ramucirumab switch maintenance (arm A) versus the continuation of first-line oxaliplatin and fluoropyrimidine (arm B) in patients with advanced human epidermal growth factor receptor 2-negative gastric or gastro-oesophageal junction cancer. We conducted a subgroup analysis aimed at assessing the differences in efficacy, safety and quality of life (QoL) according to age (<70 versus ≥70 years) and sex.
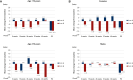
Results: No significant differences in terms of PFS (P = 0.757), OS (P = 0.588) and overall response rates (ORRs) (P = 0.238) were observed between older and younger patients. No significant differences in PFS (P = 0.646), OS (P = 0.858) and ORRs (P = 0.649) were observed between females and males. The effect of treatment arm on survival outcomes was similar across age (P = 0.094) and sex groups (P = 0.469). Looking at specific adverse events, peripheral neuropathy occurred more frequently in younger patients (P = 0.020), anaemia in older patients (P = 0.044) and hand-foot syndrome in women (P = 0.021). Older patients were less likely to receive a post-discontinuation treatment (P = 0.010), especially in arm B (P = 0.026). The impact on QoL was better in arm A, irrespective of age and sex.
Conclusion: The ARMANI trial supported the benefit of the investigated switch maintenance strategy irrespective of age and sex.
Keywords: age; gastric cancer; paclitaxel; ramucirumab; sex; switch maintenance.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Lordick F., Carneiro F., Cascinu S., et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–1020. - PubMed
-
- Rha S.Y., Oh D.Y., Yañez P., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(11):1181–1195. - PubMed
-
- Randon G., Lonardi S., Fassan M., et al. Ramucirumab plus paclitaxel as switch maintenance versus continuation of first-line oxaliplatin-based chemotherapy in patients with advanced HER2-negative gastric or gastro-oesophageal junction cancer (ARMANI): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2024;25(12):1539–1550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

