Recombinant quadrivalent influenza vaccine (RIV) induces robust cell-mediated and HA-specific B cell humoral immune responses among healthcare personnel
- PMID: 40540779
- PMCID: PMC12337806
- DOI: 10.1016/j.vaccine.2025.127361
Recombinant quadrivalent influenza vaccine (RIV) induces robust cell-mediated and HA-specific B cell humoral immune responses among healthcare personnel
Abstract
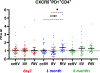
Egg-free influenza vaccines, specifically cell culture-based inactivated influenza vaccine (ccIIV) and recombinant influenza vaccine (RIV), represent a significant advancement over traditional egg-based inactivated influenza vaccines (IIV), particularly for populations with extensive vaccination histories. This comprehensive immunological study investigated the comparative efficacy of ccIIV, IIV, and RIV in healthcare personnel (HCP) with repeated vaccination histories, examining both cellular and humoral immune responses through multiple analytical approaches. Our investigation employed a multi-faceted analytical framework, combining serological assessments via hemagglutination inhibition (HI) and microneutralization (MN) assays with detailed cellular immune response analysis. We utilized advanced flow cytometry techniques with recombinant hemagglutinin (HA) probes to evaluate both circulating T follicular helper cells (cTfh) and HA-specific B cells, providing a comprehensive view of vaccine-induced immune responses. The results revealed RIV's superior immunogenicity profile, demonstrating significantly elevated levels of both cTfh and HA-specific B cells compared to ccIIV and IIV. RIV's enhanced performance was particularly evident in its response to influenza A components, with notably higher immunogenicity against both A(H3N2) and A(H1N1) strains. This superiority was reflected in elevated HI titers and markedly increased HA-specific B cell induction. While RIV also demonstrated enhanced HA-specific B cell responses against influenza B components compared to ccIIV, interestingly, HI titers remained comparable across all vaccine groups for these strains. These findings underscore the critical importance of comprehensive immune response evaluation in vaccine assessment. The disparity between cellular and serological responses, particularly for influenza HA-specific B cells, highlights that traditional serological measures alone may not fully capture the breadth and depth of vaccine-induced immunity. This study provides compelling evidence for the inclusion of cellular immunity assessments in vaccine evaluation protocols, offering crucial insights into vaccine immunogenicity that may be missed by conventional serological analysis alone.
Keywords: Cell culture derived inactivated influenza vaccine (ccIIV); Circulating follicular T helper cells (cTfh); Egg-derived inactivated influenza vaccine (IIV); HA-tags; Healthcare personnel; Influenza; Quadrivalent influenza vaccine (QIV); Recombinant influenza vaccine (RIV); Vaccine.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: A. L. N. received funding from Pfizer for an unrelated study. M. G. received funding from CDC with Ambulatory US Flu VE Network and HAIVEN (Hospitalized Adult Influenza Vaccine Effectiveness Network), outside the submitted work. M. G. received support from MedImmune/AstraZeneca for ICILE study, FDA required post-marketing LAIV4 effectiveness study in children from 2013 to 2016, outside the submitted work. All other authors report no potential conflicts. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- CDC. Past Seasons’ Vaccine Effectiveness Estimates [cited 2024 November 4]; Available from: https://www.cdc.gov/flu-vaccines-work/php/effectiveness-studies/past-sea...; 2024.
-
- Belongia EA, et al. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017;16(7):1–14. - PubMed
-
- Wilde JA, et al. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA 1999;281(10):908–13. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

