Prediction of amyloid and tau brain deposition and cognitive decline in people with Down syndrome using plasma biomarkers: a longitudinal cohort study
- PMID: 40541209
- PMCID: PMC12174012
- DOI: 10.1016/S1474-4422(25)00158-9
Prediction of amyloid and tau brain deposition and cognitive decline in people with Down syndrome using plasma biomarkers: a longitudinal cohort study
Abstract
Background: Plasma biomarkers associated with Alzheimer's disease could improve prognostic assessment for people with Down syndrome in both clinical practice and research settings. We aimed to identify the plasma biomarkers that most accurately predict longitudinal changes in Alzheimer's disease-related pathology and cognitive functioning in individuals with Down syndrome.
Methods: This longitudinal cohort study included data from 258 adults (aged ≥25 years) with Down syndrome who were followed up prospectively every 16 months as part of the longitudinal Alzheimer's Biomarker Consortium-Down Syndrome study (recruited from seven university sites in the USA and UK between July 13, 2016, and Jan 15, 2019). Participants had baseline and longitudinal assessments of plasma tau phosphorylated at threonine 217 (p-tau217), glial fibrillary acidic protein (GFAP), amyloid β (Aβ)42/40, neurofilament light (NfL), or total tau (t-tau). Associations of baseline plasma biomarkers and longitudinal changes in plasma biomarkers with changes in global cognitive functioning (Down Syndrome Mental Status Examination [DS-MSE] scores), Aβ-PET, and tau-PET were examined using linear regression models. Plasma biomarker-associated risk of progression to dementia was assessed using Cox regression analysis.
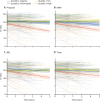
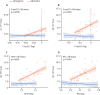
Findings: Baseline p-tau217, as well as GFAP, NfL, or t-tau, were individually associated with longitudinal changes in DS-MSE, Aβ-PET, and tau-PET, and with progression to dementia. However, in combined models, only baseline p-tau217 remained associated with changes in DS-MSE (β -0·30 [95% CI -0·45 to -0·15], p=0·0001, n=220), tau-PET (0·42 [0·14 to 0·70], p=0·0039, n=88), and progression to dementia (hazard ratio 3·51 [95% CI 1·76-7·00], p=0·0004, n=194), whereas baseline p-tau217 (0·29 [0·14-0·45], p=0·0003) and GFAP (0·37 [0·18-0·56], p=0·0003) were associated with changes in Aβ-PET (n=106 for both). Similar associations were shown between longitudinal p-tau217 or GFAP and changes in DS-MSE (p-tau217: β -0·33 [95% CI-0·52 to -0·13], p=0·0015, n=133), tau-PET (p-tau217: 0·61 [0·40 to 0·83], p<0·0001, n=87), and Aβ-PET (p-tau217: 0·35 [0·19 to 0·50], p<0·0001; GFAP: 0·49 [0·27 to 0·70], p<0·0001, n=88).
Interpretation: Baseline and longitudinal plasma p-tau217 were associated with subsequent decline in global cognition, progression to dementia, and increased tau burden, whereas baseline p-tau217 and GFAP were associated with Aβ accumulation. These findings suggest that plasma p-tau217 and GFAP might be valuable for prognostic assessment of Alzheimer's disease in people with Down syndrome in both clinical and research contexts. The results further support evaluation of these biomarkers for monitoring disease progression in clinical trials of Down syndrome-related Alzheimer's disease.
Funding: The European Research Council and National Institute on Aging (National Institute of Health).
Copyright © Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND license.
Conflict of interest statement
Declaration of interests LEC has received research support from GE Healthcare, Life Molecular Imaging, and Springer Healthcare (funded by Eli Lilly), both paid to their institution. LEC's salary is supported by the MSCA postdoctoral fellowship research grant (grant number 101108819) and the Alzheimer Association Research Fellowship grant (grant number 23AARF-1029663). NM-C has received consultancy fees from Biogen, Eli Lilly, Merck, and Owkin in the past 2 years. SJ reports grants from Swedish Alzheimer Foundation, Kockska Foundations, and Foundation for Gamla Tjänarinnor. BMA, CML, and EH report funding from the NIH. EH reports funding from the Brightfocus and consulting fees from Cyclo Therapeutics, Alzheon, and Elsevier. SLH reports consulting from Ionis Pharmauticals and being a Chair of Alzheimer's Association Down Syndrome Professional Interest Area. SHZ reports funding from Cambridgeshire & Peterborough Foundation NHS Trust, UK and support for attending meetings and travel. MM reports royalties from University of Rochester, consulting fees from NovoGlia and Ireneo Health, and several US patents. BDC reports support from Alzheimer's Disease Research Centers Program, Eunice Kennedy Shriver Intellectual and Developmental Disabilities Research Centers Program, National Center for Advancing Translational Sciences, National Centralized Repository for Alzheimer Disease and Related Dementias, DS-Connect (The Down Syndrome Registry), NIHR Cambridge Biomedical Research Centre, Windsor Research Unit, CPFT, and Fulbourn Hospital Cambridge, UK and consulting fees from Alnylam. SLH reports consulting from Ionis Pharmauticals. BLH reports receipt of speaker's fees, royalties from two books. RO received research funding and support from Avid Radiopharmaceuticals, Janssen Research & Development, Roche, Quanterix, and Optina Diagnostics; has given lectures in symposia sponsored by GE Healthcare; received speaker fees from Springer; is an advisory board member for Asceneuron; and is a steering committee member for Biogen and Bristol Myers Squibb; all the aforementioned funding has been paid to his institutions. OH is an employee of Lund University and Eli Lilly. All other authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

