Sociodemographic, Clinical, and Psychosocial Predictors of Short- and Long-term Study Retention in the Diabetes Prevention Program (DPP) Outcomes Study (DPPOS)
- PMID: 40541257
- PMCID: PMC12451849
- DOI: 10.2337/dc25-0520
Sociodemographic, Clinical, and Psychosocial Predictors of Short- and Long-term Study Retention in the Diabetes Prevention Program (DPP) Outcomes Study (DPPOS)
Abstract
Objective: Success of longitudinal studies depends on retention of participants. We examined characteristics as predictors of retention among participants with prediabetes and type 2 diabetes (T2D) in the Diabetes Prevention Program (DPP) and the follow-up DPP Outcomes Study.
Research design and methods: A total of 3,234 adults at high risk of T2D joined the DPP (1996-1999, mean age 51 ± 10 years). They were randomized to lifestyle, metformin, or placebo intervention, and then followed through 2020. Logistic regression models estimated the association between baseline sociodemographic, clinical and psychosocial characteristics (life events, family functioning, social support), and short-term retention (∼3 years). Cox proportional hazards models, censoring at death, estimated the association between baseline and time-varying characteristics and time to dropout over the entire 20 years of follow-up.
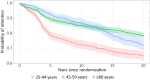
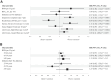
Results: Among surviving participants (n = 3,218), 93% were retained after 3 years, and 75% of those surviving remained engaged over 20 years. Younger age was associated with dropout during DPP and over 20 years of follow-up. Female sex, non-White race and ethnicity, employment, and lack of baseline depressive symptoms were associated with better long-term retention. Over time, better health state (SF-36) (hazard ratio [HR]: 0.89 per 0.1 point; 95% CI: 0.83-0.95) was associated with retention. Greater BMI (HR: 1.06 per 5 kg/m2; 95% CI: 1.00-1.12), more recent life events (HR: 1.08; 95% CI: 1.02-1.14), and depressive symptoms (HR: 1.11 per 5 points; 95% CI: 1.05-1.18) were associated with reduced retention. Among adults 45-59 years of age at baseline, development of T2D was associated with better retention (HR: 0.75; 95% CI: 0.58-0.97).
Conclusions: Twenty-year retention of a racially and geographically diverse cohort with prediabetes is possible. Retention was associated with age, psychosocial factors, T2D development, and BMI.
© 2025 by the American Diabetes Association.
Conflict of interest statement
Figures

References
-
- Fewtrell MS, Kennedy K, Singhal A, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child 2008;93:458–461 - PubMed
-
- Furlan AD, Pennick V, Bombardier C, van Tulder M, Editorial Board, Cochrane Back Review Group . 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 2009;34:1929–1941 - PubMed
-
- Walsh M, Devereaux PJ, Sackett DL.. Clinician trialist rounds: 28. When RCT participants are lost to follow-up. Part 1: why even a few can matter. Clin Trials 2015;12:537–539 - PubMed
-
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–1064 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK048375/DK/NIDDK NIH HHS/United States
- U01 DK048434/DK/NIDDK NIH HHS/United States
- U01 DK048413/DK/NIDDK NIH HHS/United States
- U01 DK048397, U01 DK048412, U01 DK048404/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- U01 DK048339/DK/NIDDK NIH HHS/United States
- U01 DK048349, U01 DK048381, U01 DK048468/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- U01 DK048514, U01 DK048437, U01 DK048413/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- U01 DK048468/DK/NIDDK NIH HHS/United States
- U01 DK048387/DK/NIDDK NIH HHS/United States
- U01 DK048404/DK/NIDDK NIH HHS/United States
- U01 DK048377/DK/NIDDK NIH HHS/United States
- U01 DK048407/DK/NIDDK NIH HHS/United States
- U01 DK048437/DK/NIDDK NIH HHS/United States
- U01 DK048406/DK/NIDDK NIH HHS/United States
- U01 DK048411, U01 DK048406, U01 DK048380/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- U01 DK048412/DK/NIDDK NIH HHS/United States
- U19 AG078558/AG/NIA NIH HHS/United States
- U01 DK048489, U01 DK048339, U01 DK048377,/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- U01 DK048434, U01 DK048485, U01 DK048375/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- U01 DK048397/DK/NIDDK NIH HHS/United States
- U01 DK048381/DK/NIDDK NIH HHS/United States
- U01 DK048514/DK/NIDDK NIH HHS/United States
- U01 DK048485/DK/NIDDK NIH HHS/United States
- U01 DK048411/DK/NIDDK NIH HHS/United States
- U01 DK048387, U01 DK048407, U01 DK048443/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- U01 DK048443/DK/NIDDK NIH HHS/United States
- U01 DK048380/DK/NIDDK NIH HHS/United States
- U01 DK048400/DK/NIDDK NIH HHS/United States
- U01 DK048489/DK/NIDDK NIH HHS/United States
- U01 DK048349/DK/NIDDK NIH HHS/United States
- U01 DK048400/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

