MyD88 signalling in B cells and antibody responses during Oropouche virus-induced neurological disease in mice
- PMID: 40541539
- PMCID: PMC12278419
- DOI: 10.1016/j.ebiom.2025.105815
MyD88 signalling in B cells and antibody responses during Oropouche virus-induced neurological disease in mice
Abstract
Background: Oropouche virus (OROV) is a neglected insect-borne orthobunyavirus that causes a febrile illness, neurological disease, and pregnancy complications in humans across an endemic area spanning South and Central America. The host factors associated with disease pathogenesis have nonetheless remained obscure, and little is known about the immune determinants of protection against OROV.
Methods: We tracked morbidity, mortality, viral loads, and serum neutralisation in wild-type (WT), Rag1-/-, CD19-Cre+Ifnarfl, and CD19-Cre+MyD88fl mice and performed immunophenotyping experiments, passive serum transfers, and adoptive cell transfers to determine how early antibody responses and B cell subsets control viral replication and dissemination to the central nervous system after infection with OROV.
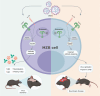
Findings: In line with a protective role for B cells, WT mice efficiently produced OROV-specific antibodies within 6 days of infection. Serum transfer containing neutralising IgM from WT to Rag1-/- mice prevented neurological disease in OROV-challenged mice. CD19-Cre+MyD88fl mice but not CD19-Cre+Ifnarfl mice were vulnerable to neurological disease and produced lower titres of OROV-specific antibodies that exhibited suboptimal neutralisation and potency compared with MyD88-sufficient mice. CD19-Cre+MyD88fl mice also presented with reduced numbers of marginal zone B (MZB) cells and plasmablasts after infection, which were associated with high viral burdens and lethality. Adoptive transfer of MZB cells from WT mice protected CD19-Cre+MyD88fl mice and partially protected Rag1-/- mice from lethal infection with OROV.
Interpretation: Early MyD88 signalling in B cells is required for optimal antibody responses that limit viral replication and neurological disease in mice infected with OROV.
Funding: São Paulo Research Foundation (FAPESP), National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES), Unicamp Research Affairs Office, PIPAE University of São Paulo, Wellcome Trust, and National Institute of Science and Technology on Photonics Applied to Cell Biology (INFABIC, Unicamp).
Keywords: Emerging viruses; IgM; Innate immune response; Marginal zone B cells; Oropouche virus; Vector-borne diseases.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests MSD is an advisor or consultant for Moderna, Ocugen, Topspin Therapeutics, IntegerBio, MacroGenics, Inbios, Akagera Medicines, Merck, Bavarian Nordic, GlaxoSmithKline, and Vir Biotechnology. MSD has received unrelated funding via sponsored research agreements from Moderna, Emergent BioSolutions, Bavarian Nordic, and Vir Biotechnology. DAP has received unrelated funding via competitive awards from the Medical Research Council, the Open Medicine Foundation, the PolyBio Research Foundation, and the National Institute for Health Research.
Figures





References
-
- Pinheiro F.D., Pinheiro M., Bensabath G., Causey O.R., Shope R.E. Epidemia de vírus Oropouche em Belém. Rev Ser Esp Saude Pub. 1962;12:15–23.
-
- Ministério da Saúde Oropouche. https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/o/oropouche/paine...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

