Virus-Induced Cellular Senescence Causes Pulmonary Sequelae Post-Influenza Infection
- PMID: 40541563
- PMCID: PMC12419848
- DOI: 10.1111/acel.70140
Virus-Induced Cellular Senescence Causes Pulmonary Sequelae Post-Influenza Infection
Abstract
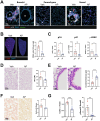
Influenza A virus (IAV) infection causes acute and long-term lung damage. Here, we used immunostaining, genetic, and pharmacological approaches to determine whether IAV-induced cellular senescence causes prolonged alterations in lungs. Mice infected with a sublethal dose of H1N1p2009 exhibited cellular senescence, as evidenced by increased pulmonary expression of p16, p21, β-galactosidase and the DNA damage marker gamma-H2A.X. Cellular senescence began 4 days post-infection (dpi) in the bronchial epithelium, then spread to the lung parenchyma by 7 and 28 dpi (long after viral clearance), and then declined by 90 dpi. At 28 dpi, the lungs showed severe remodeling with structural bronchial and alveolar lesions, abrasion of the airway epithelium, and pulmonary emphysema and fibrotic lesions that persisted up to 90 dpi. In mice and nonhuman primates, persistence of senescent cells in the bronchial wall on 28 dpi was associated with abrasion of the airway epithelium. In p16-ATTAC mice, depletion of p16-expressing cells with AP20187 reduced pulmonary emphysema and fibrosis and led to complete recovery of the airway epithelium at 28 dpi, indicating a marked acceleration of the epithelial repair process. Treatment with the senolytic drug ABT-263 also accelerated epithelial repair without affecting pulmonary fibrosis or emphysema. These positive effects occurred independently of viral clearance and lung inflammation at 7 dpi. Finally, AP20187 treatment of p16-ATTAC mice at 15 dpi led to complete recovery of the airway epithelium at 28 dpi. Thus, virus-induced senescent cells contribute to the pulmonary sequelae of influenza; targeting senescent cells may represent a new preventive therapeutic option.
Keywords: acute response; cellular senescence; chronic damage; epithelial damage; fibrosis; genetic approach; influenza; senolysis.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Barnes, P. J. , Baker J., and Donnelly L. E.. 2019. “Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases.” American Journal of Respiratory and Critical Care Medicine 200: 556–564. - PubMed
-
- Born, E. , Lipskaia L., Breau M., et al. 2023. “Eliminating Senescent Cells Can Promote Pulmonary Hypertension Development and Progression.” Circulation 147: 650–666. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

