TFAP2C drives cisplatin resistance in bladder cancer by upregulating YAP and activating β-catenin signaling
- PMID: 40541807
- PMCID: PMC12284524
- DOI: 10.1016/j.jbc.2025.110387
TFAP2C drives cisplatin resistance in bladder cancer by upregulating YAP and activating β-catenin signaling
Abstract
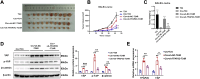
Cisplatin-based chemotherapy is a conventional therapy for muscle-invasive bladder cancer (BC); however, its efficacy is often limited by the emergence of resistance to cisplatin. Yes-associated protein (YAP) and β-catenin are involved in this resistance, yet their upstream regulators are not well defined. This study investigates the role of TFAP2C in regulating YAP expression and its impact on cisplatin resistance in BC. The Cancer Genome Atlas (TCGA) gene expression data and GSE231835 dataset were analyzed to identify potential transcription factors regulating YAP. Assessed TFAP2C and YAP expression in clinical samples and cell lines. Functional assays were performed following TFAP2C knockdown. Dual-luciferase reporter assays and Chromatin immunoprecipitation (ChIP) confirmed TFAP2C binding to the YAP promoter. A mouse model evaluated the effects of TFAP2C silencing on tumor growth and cisplatin resistance. The results showed that TFAP2C was identified as an upstream activator of YAP, with elevated expression in cisplatin-resistant BC cell lines and positive correlation with YAP expression. Silencing TFAP2C reduced malignant behaviors, decreased YAP, phosphorylated YAP (p-YAP) and β-catenin levels, and increased apoptosis in both cisplatin-sensitive and cisplatin-resistant BC cells. Besides, TFAP2C directly binds to the YAP promoter, enhancing its transcription. In the xenograft model, TFAP2C silencing significantly inhibited tumor growth and reduced cisplatin resistance. TFAP2C promotes cisplatin resistance and malignant behavior in BC by upregulating YAP and activating the β-catenin signaling pathway. Targeting TFAP2C offers a novel therapeutic strategy to overcome cisplatin resistance in BC, representing a new discovery in combating chemoresistance.
Keywords: YAP; bladder cancer; cisplatin resistance; transcription regulation; β-catenin.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures







Similar articles
-
Reversal of cisplatin resistance in oral squamous cell carcinoma by piperlongumine loaded smart nanoparticles through inhibition of Hippo-YAP signaling pathway.Transl Res. 2024 Jun;268:63-78. doi: 10.1016/j.trsl.2024.03.004. Epub 2024 Mar 16. Transl Res. 2024. PMID: 38499286
-
KHSRP promotes the malignant behavior and cisplatin resistance of bladder cancer cells through the CLASP2/MAPRE1 axis.Pharmacogenomics J. 2025 May 17;25(3):14. doi: 10.1038/s41397-025-00374-1. Pharmacogenomics J. 2025. PMID: 40382315
-
KLF5-regulated ZDHHC8 reduces chemosensitivity in high-grade serous ovarian cancer through β-catenin signaling.Sci Rep. 2025 Jul 18;15(1):26176. doi: 10.1038/s41598-025-11845-7. Sci Rep. 2025. PMID: 40681764 Free PMC article.
-
LIMA1 inhibits cisplatin resistance and malignant biological behavior of bladder cancer cells by suppressing the Wnt/β-catenin pathway.BMC Med Genomics. 2025 Apr 23;18(1):78. doi: 10.1186/s12920-025-02146-z. BMC Med Genomics. 2025. PMID: 40269880 Free PMC article.
-
YAP as a therapeutic target in esophageal squamous cell carcinoma: insights and strategies.Ann Med. 2025 Dec;57(1):2536200. doi: 10.1080/07853890.2025.2536200. Epub 2025 Jul 22. Ann Med. 2025. PMID: 40693409 Free PMC article. Review.
Cited by
-
Verteporfin inhibits the cGAS-STING pathway and improves the tumor microenvironment during cisplatin treatment in hepatocellular carcinoma.Front Immunol. 2025 Aug 22;16:1658042. doi: 10.3389/fimmu.2025.1658042. eCollection 2025. Front Immunol. 2025. PMID: 40918140 Free PMC article.
References
-
- Li F., Zheng Z., Chen W., Li D., Zhang H., Zhu Y., et al. Regulation of cisplatin resistance in bladder cancer by epigenetic mechanisms. Drug Resist Updat. 2023;68 - PubMed
-
- Li F., Zhang H., Huang Y., Li D., Zheng Z., Xie K., et al. Single-cell transcriptome analysis reveals the association between histone lactylation and cisplatin resistance in bladder cancer. Drug Resist Updat. 2024;73 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

