Yttrium oxide nanoparticles induce selective cytotoxicity, genomic instability and ROS mitochondrial P53 mediated apoptosis in human pancreatic cancer cells
- PMID: 40542009
- PMCID: PMC12181288
- DOI: 10.1038/s41598-025-05088-9
Yttrium oxide nanoparticles induce selective cytotoxicity, genomic instability and ROS mitochondrial P53 mediated apoptosis in human pancreatic cancer cells
Abstract
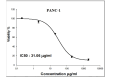
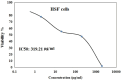
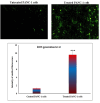
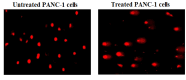
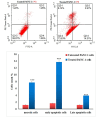
Pancreatic cancer is a hard-to-treat tumor with a poor prognosis. While traditional pancreatic cancer therapies can be effective, issues like cytotoxicity, low selectivity, and drug resistance still pose major challenges. Nanotechnology has shown promise in improving cancer diagnosis and treatment. Yttrium oxide nanoparticles (Y2O3-NPs), for example, have demonstrated potent selective cytotoxicity against triple negative breast cancer cells; but their effects on pancreatic cancer cells have not been explored. This study aimed to explore the impact of Y2O3-NPs on cell proliferation, DNA integrity, and oxidative stress in pancreatic cancer (PANC-1) and human skin fibroblast (HSF) cells. The cytotoxicity of Y2O3-NPs after 72 h were estimated using Sulforhodamine (SRB) cytotoxicity assay, while alkaline Comet assay was done to study genomic DNA integrity. Generation level of reactive oxygen species (ROS) and integrity of mitochondrial membrane potential were also analyzed. Apoptosis induction was investigated using Flow Cytometry and expression level of apoptotic (p53), anti-apoptotic (Bcl2) and mitochondrial (ND3) genes was measured using quantitative RTPCR. Our findings exhibited that Y2O3-NPs had strong selective cytotoxicity against PANC-1 cells with an IC50 value of 31.06 µg/ml, while having minimal effect on normal HSF cells (IC50 = 319.21 µg/ml). Treatment of PANC-1 cells with Y2O3-NPs at the IC50 concentration for 72 h significantly increased intracellular ROS levels and DNA damage, along with a notable reduction in mitochondrial membrane potential. Additionally, a significant rise in necrotic, early, and late apoptotic cells was observed, accompanied by downregulation of the anti-apoptotic Bcl2 gene and upregulation of the apoptotic p53 and mitochondrial ND3 genes. These findings highlight the selective toxicity of Y2O3-NPs towards cancerous PANC-1 cells, with minimal impact on normal cells. Y2O3-NPs appear to induce apoptosis in cancer cells by increasing ROS generation, damaging DNA, disrupting mitochondrial function, and triggering cell death. This study suggests that Y2O3-NPs may be a promising candidate for pancreatic cancer treatment. Further research is needed to fully explore their therapeutic potential.
Keywords: Apoptosis; Cytotoxicity; DNA damage; Mitochondrial integrity; Pancreatic cancer cancers; ROS; Y2O3-NPs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

