Functional transposition of renal functions to the posterior intestine during maturation in male three-spined stickleback
- PMID: 40542020
- PMCID: PMC12181344
- DOI: 10.1038/s41598-025-05513-z
Functional transposition of renal functions to the posterior intestine during maturation in male three-spined stickleback
Abstract
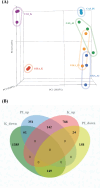
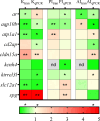
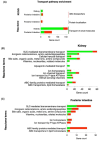
During the breeding season, the male stickleback proximal tubule of the kidney undergoes hypertrophy. This is due to the synthesis of the nest building protein spiggin, in response to increased levels of 11-ketotestosterone. The increased protein synthesis that is initiated during breeding alters the kidney function and the ability to secrete excess water, to osmoregulate, in fresh water. It has earlier been shown that there exist organ specific differences in transport proteins between mature and non-mature three-spined stickleback. To understand the molecular mechanisms compensating for kidney functions, this study examined transport genes responsible for functional changes between the kidney and intestine. RNA sequencing was performed on castrated and 11-ketoandrostenedione (11KA)-treated male stickleback. Results showed organ-specific responses: 2,549 differentially expressed genes (DEGs) in the kidney and 885 in the posterior intestine, with 210 shared between the organs. Solute transporters, aquaporin 10a and cadherin-17, were upregulated in the posterior intestine but downregulated in the kidney in 11KA treated males. Enrichment analysis revealed distinct biological processes, primarily involving solute transporters, indicating functional adaptation. While amino acid and ion transport were downregulated in the kidney, compensatory transport was observed in the posterior intestine. However, cellular hexose transporters were downregulated in both organs, suggesting a reduction in glucose absorption and passive water diffusion. The present study shows that androgens alter the expression of cellular transporters and redirect functions of the kidney to the posterior intestine. The results also indicate reduced glucose absorption in breeding, male three-spined stickleback.
Keywords: 11-ketoandrostenedione; Androgen; Glucose; Kidney; Solute transporters.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Effectiveness and safety of vitamin D in relation to bone health.Evid Rep Technol Assess (Full Rep). 2007 Aug;(158):1-235. Evid Rep Technol Assess (Full Rep). 2007. PMID: 18088161 Free PMC article.
-
Endovascular treatment for ruptured abdominal aortic aneurysm.Cochrane Database Syst Rev. 2017 May 26;5(5):CD005261. doi: 10.1002/14651858.CD005261.pub4. Cochrane Database Syst Rev. 2017. PMID: 28548204 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Sexual maturation and changes in water and salt transport components in the kidney and intestine of three-spined stickleback (Gasterosteus aculeatus L.).Comp Biochem Physiol A Mol Integr Physiol. 2015 Oct;188:107-19. doi: 10.1016/j.cbpa.2015.06.021. Epub 2015 Jun 29. Comp Biochem Physiol A Mol Integr Physiol. 2015. PMID: 26135640
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.Health Technol Assess. 2006 Dec;10(49):iii-iv, ix-xi, 1-157. doi: 10.3310/hta10490. Health Technol Assess. 2006. PMID: 17134597
References
-
- Bell, M. A. & Foster, S. A. The Evolutionary Biology of the Threespine Stickleback (Oxford University Press, 1994).
-
- Ostlund-Nilsson, S., Mayer, I. & Huntingford, F. A. Biology of the three-spined Stickleback (CRC, 2006).
-
- De Ruiter, A. J. H. Changes in glomerular structure after sexual maturation and seawater adaptation in males of the Euryhaline teleost Gasterosteus aculeatus L. Cell. Tissue Res.206, 1–20. 10.1007/bf00233603 (1980). - PubMed
-
- Páll, M. K. et al. Changes in reproductive physiology and behaviour over the nesting cycle in male three-spined sticklebacks. J. Fish. Biol.66, 1400–1410. 10.1111/j.0022-1112.2005.00691.x (2005).
-
- Haley, A. L., Dalziel, A. C. & Weir, L. K. A comparison of nuptial coloration and breeding behaviour in white and common marine threespine stickleback (Gasterosteus aculeatus) ecotypes. Evol. Ecol. Res.20, 145–166 (2019).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

