Wavelength-dependent photodissociation of iodomethylbutane
- PMID: 40542041
- PMCID: PMC12181341
- DOI: 10.1038/s41598-025-04905-5
Wavelength-dependent photodissociation of iodomethylbutane
Abstract
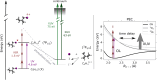
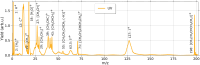
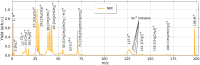
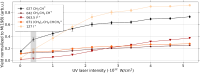
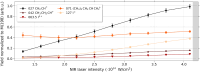
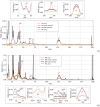
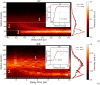
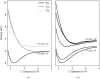
Ultrashort XUV pulses of the Free-Electron-LASer in Hamburg (FLASH) were used to investigate laser-induced fragmentation patterns of the prototypical chiral molecule 1-iodo-2-methyl-butane ([Formula: see text] [Formula: see text]I) in a pump-probe scheme. Ion velocity-map images and mass spectra of optical-laser-induced fragmentation were obtained for subsequent FEL exposure with photon energies of 63 eV and 75 eV. These energies specifically address the iodine 4d edge of neutral and singly charged iodine, respectively. The presented ion spectra for two optical pump-laser wavelengths, i.e., 800 nm and 267 nm, reveal substantially different cationic fragment yields in dependence on the wavelength and intensity. For the case of 800-nm-initiated fragmentation, the molecule dissociates notably slower than for the 267 nm pump. The results underscore the importance of considering optical-laser wavelength and intensity in the dissociation dynamics of this prototypical chiral molecule that is a promising candidate for future studies of its asymmetric nature.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Young, L. et al. Roadmap of ultrafast X-ray atomic and molecular physics. J. Phys. B At. Mol. Opt. Phys.51, 032003 (2018).
-
- McNeil, B. & Thompson, N. X-ray free-electron lasers. Nat. Photon.4, 814–821 (2010).
-
- Emma, P. et al. First lasing and operation of an ångstrom-wavelength free-electron laser. Nat. Photon.4, 641–647 (2010).
-
- Hartmann, G. et al. Attosecond time–energy structure of X-ray free-electron laser pulses. Nat. Photon.12, 215–220 (2018).
-
- Erk, B. et al. Imaging charge transfer in iodomethane upon X-ray photoabsorption. Science345, 288 (2014). - PubMed
LinkOut - more resources
Full Text Sources

