Ag₃PO₄@ZnO kraft lignin composite for optimized photocatalytic degradation of methylene blue using response surface methodology
- PMID: 40542064
- PMCID: PMC12181256
- DOI: 10.1038/s41598-025-05597-7
Ag₃PO₄@ZnO kraft lignin composite for optimized photocatalytic degradation of methylene blue using response surface methodology
Abstract
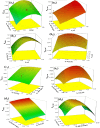
This research explores the use of kraft lignin (KL), derived from pulping black liquor waste, as a supportive medium for Ag3PO4@ZnO (AZ-NC) p-n heterojunction and design a new cost-effective ternary KL-Ag3PO4@ZnO nanocomposite (AZKL). The aim is to improve its photocatalytic efficiency in treating textile wastewater while tackling environmental issues such as chemical stability, charge carrier separation, and the production of secondary waste during the photocatalytic process. The response surface methodology (RSM) analysis shows that AZKL is highly effective catalyst for methylene blue (MB: 10 - 25 mg/L) dye mineralization, achieving a rapid decolorization (> 98.2% within 40 min) under visible light at a near-neutral pH (7.48) with maintained high catalytic activity across four consecutive cycles. This outstanding performance is driven by the synergistic interplay of AZKL-based photocatalysis and advanced oxidation process using 0.03% H2O2 co-catalyst. Gas chromatography-mass spectrometry analysis reveals that MB dye degrades stepwise into intermediates such as N, N-dimethyl-p-phenylenediamine, hydroquinone, and formic acid, ultimately mineralizing completely into CO₂ and H₂O. The dominant reactive oxygen species driven this multi-step process are identified as hydroxyl radicals (•OH) and photogenerated holes (h⁺), with H₂O₂ and superoxide radicals (•O₂⁻) playing secondary roles. The data also highlights the multifunctional role of KL support, which enhances charge carrier separation, captures dye molecules, and prevents Zn2+/Ag+ ion leaching (less than 0.2 ppm) into the treated water during photocatalysis. This is facilitated by the electron-donating polyphenolic hydroxyl groups on the KL surface, which reduce Ag⁺ to metallic silver and stabilize AZ-NC heterojunction under light irradiation, creating Schottky junctions that improve charge transfer efficiency while reducing secondary contamination risks. A practical case study further illustrates the effectiveness of AZKL in treating real textile effluents, as evidenced by the improved biodegradability of residual organic matter, indicated by changes in chemical/biological oxygen demands (COD/BOD) ratios from 2.62 to 1.47 and inhibition tests against E. coli, meeting wastewater discharge standards. The findings emphasize that the AZKL composite could serve as an effective and adaptable photocatalyst for breaking down organic pollutants and treating intricate wastewater systems.
Keywords: Ag3PO4@ZnO p-n heterojunction; Kraft lignin; Response surface methodology; Textile wastewater treatment; Waste valorization.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





References
-
- Lanjwani, M. F., Tuzen, M., Khuhawar, M. Y. & Saleh, T. A. Trends in photocatalytic degradation of organic dye pollutants using nanoparticles: A review. Inorg. Chem. Commun.159, 111613 (2024).
-
- Kulis-Kapuscinska, A. et al. Photocatalytic degradation of methylene blue at nanostructured ZnO thin films. Nanotechnology34, 155702 (2023). - PubMed
-
- Geed, S. R., Samal, K. & Tagade, A. Development of adsorption-biodegradation hybrid process for removal of methylene blue from wastewater. J. Environ. Chem. Eng.7, 103439 (2019).
-
- Oladoye, P. O., Ajiboye, T. O., Omotola, E. O. & Oyewola, O. J. Methylene blue dye: toxicity and potential elimination technology from wastewater. Results Eng.16, 100678 (2022).
-
- Khan, I. et al. Review on methylene blue: its properties, uses, toxicity and photodegradation. Water14, 242 (2022).
LinkOut - more resources
Full Text Sources
Miscellaneous

