Birth mode is associated with layer-specific mechanical changes in fetal membranes
- PMID: 40542102
- PMCID: PMC12181243
- DOI: 10.1038/s41598-025-04752-4
Birth mode is associated with layer-specific mechanical changes in fetal membranes
Abstract
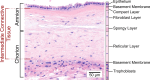
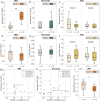
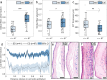
Rupture of fetal membranes and subsequent full-term birth are prerequisites for neonatal health, and a preterm rupture can lead to life-threatening complications. Our study determines the mechanical properties of term fetal membranes to identify perinatal structural changes by a unique biophysical multiscale approach, including atomic force microscopy, shear rheology, tabletop magnetic resonance elastography (MRE), and high-resolution optical microscopy. Fetal membranes from term spontaneous vaginal deliveries were compared to those from primary cesarean sections, used as a control group for pre-labor membranes. Spontaneously delivered term fetal membranes are softer and easier to deform in MRE experiments (median stiffness: 1.9 kPa, IQR 1.6-2.4) compared to controls (4.7 kPa, IQR 3.8-5.6); p < 0.001) and show increased water diffusion (median: 1.78 × 10-3 mm2/s, IQR: (1.65-1.84) × 10-3 vs. 1.66 × 10-3 mm2/s, IQR (1.60-1.73) × 10-3; p = 0.047). Their intermediate connective tissue layer (i.e. the collagen-rich area enclosed by the amnion and chorion) exhibits less ordered fiber alignment (median order parameter: 0.52, IQR 0.44-0.58 vs. 0.55, IQR 0.47-0.62; p = 0.04) and a looser fiber structure, as indicated by a significantly lower fiber area fraction (median: 0.33, IQR 0.25-0.46 vs. 0.73, IQR 0.63-0.88; p < 0.001) compared to the control membranes. These layer-specific changes in both structure and viscoelasticity are evidence for the dominant role of the intermediate connective tissue in maintaining membrane stability and the onset of rupture. Our mechanical and histopathological findings highlight the potential of mechanics-based screening-methods to assess the risk of preterm rupture and preterm birth to reduce neonatal morbidity.
Keywords: Atomic force microscopy; Fetal membranes; Magnetic resonance elastography; Rupture of membranes; Shear rheology; Tissue viscoelasticity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and the use of human tissue samples: This study was approved by the local ethics committee (Approval No. 540/21-ek, Date: 2021-12-20). All experiments were performed in accordance with relevant guidelines and regulations. Human fetal membranes (FMs) were collected from pre-selected term pregnancies. All participants were provided informed consent during the study.
Figures

Similar articles
-
Planned early birth versus expectant management (waiting) for prelabour rupture of membranes at term (37 weeks or more).Cochrane Database Syst Rev. 2017 Jan 4;1(1):CD005302. doi: 10.1002/14651858.CD005302.pub3. Cochrane Database Syst Rev. 2017. PMID: 28050900 Free PMC article.
-
Fetal membrane imaging and the prediction of preterm birth: a systematic review, current issues, and future directions.BMC Pregnancy Childbirth. 2016 Dec 9;16(1):387. doi: 10.1186/s12884-016-1176-5. BMC Pregnancy Childbirth. 2016. PMID: 27938341 Free PMC article.
-
Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks' gestation for improving pregnancy outcome.Cochrane Database Syst Rev. 2017 Mar 3;3(3):CD004735. doi: 10.1002/14651858.CD004735.pub4. Cochrane Database Syst Rev. 2017. PMID: 28257562 Free PMC article.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease.Cochrane Database Syst Rev. 2017 Nov 2;11(11):CD012847. doi: 10.1002/14651858.CD012847. Cochrane Database Syst Rev. 2017. PMID: 29095502 Free PMC article.
References
-
- Zhu, C. et al. Nutritional and physiological regulation of water transport in the conceptus. Adv. Exp. Med. Biol.1354, 109–125 (2022). - PubMed
-
- Moore, R. M., Mansour, J. M., Redline, R. W., Mercer, B. M. & Moore, J. J. The physiology of fetal membrane rupture: Insight gained from the determination of physical properties. Placenta27, 1037–1051 (2006). - PubMed
-
- Friel, N. A. et al. Amniotic fluid, cells, and membrane application. Oper. Tech. Sports Med.25, 20–24 (2017).
-
- Mercer, B. M. Preterm premature rupture of the membranes. Obstet. Gynecol.101, 178–193 (2003). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

