Impacts of commercial bile acids on growth performance, immune responses and expression genes of lipid metabolism in Nile tilapia fingerlings Oreochromis niloticus
- PMID: 40542118
- PMCID: PMC12181359
- DOI: 10.1038/s41598-025-06813-0
Impacts of commercial bile acids on growth performance, immune responses and expression genes of lipid metabolism in Nile tilapia fingerlings Oreochromis niloticus
Abstract
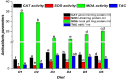
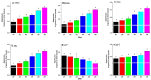
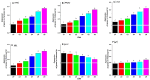
The current investigation evaluated the impact of the dietary addition of commercial bile acids (BAs) on growth, nutrient assimilation, immunity, antioxidant status, intestinal and hepatic histomorphometry, and gene expression of lipid metabolism in Nile tilapia (Oreochromis niloticus). In a study conducted for seventy days, 180 healthy fingerlings weighing 9 ± 0.5 g were divided into 18 hapas measuring 0.7 × 0.7 × 1.0 m. The fish were fed on six meals enriched with varied amounts of BAs: 0.0 (D1), 0.1 (D2), 0.2 (D3), 0.3 (D4), 0.4 (D5), and 0.5 (D6) g/kg diet. Nile tilapia fed the D3 diet exhibited significantly enhanced growth performance, with a specific growth rate of 1.89%/day and had the greatest feed conversion ratio (1.25), protein productive value, and energy utilization (33.28%). Fish fed the D3 exhibited significantly the highest crude protein content (64.50%). Energy content varied significantly, with D1 showing the lowest value (533.34 Kcal/100 g) and D3 the highest (604.27 Kcal/100 g). D3 improved biochemical indicators, immunological parameters, and digestive enzymes of O. niloticus. Histological analysis revealed notable liver and intestinal integrity enhancements among fish receiving BA-enriched diets, especially D3. Additionally, gene expression related to lipid metabolism in liver, peritoneal fat, and muscle tissues was upregulated in the treatment groups, especially 0.2 g/kg BAs compared to the control group. Results illustrate significant modulation of lipid metabolism gene expression parameters (Adipose triglyceride lipase; ATGL, Hormone-sensitive lipase; HSL, Peroxisome proliferator-activated receptor α; PPARα, Fatty acid synthase; FAS) by BAs treatments and were upregulated in BA-fed groups (D2-D6). Conversely, Carnitine palmitoyl transferase 1; CPT-1and Insulin-like growth factor-II; Igf-II expression declined, particularly when the BAs dose was increased. Accordingly, dietary 0.2 g/kg BAs supplementation positively influences on physiological, biochemical parameters, and lipid metabolic of Nile tilapia, making it a promising feed additive for aquaculture.
Keywords: Antioxidants; Digestive enzyme; Intestinal and liver morphology; Intestinal microbiota.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Dietary corn silk enhances growth, immunity, and gene expression in Nile tilapia (Oreochromis niloticus) cultured in a biofloc system.Fish Shellfish Immunol. 2025 Oct;165:110555. doi: 10.1016/j.fsi.2025.110555. Epub 2025 Jul 9. Fish Shellfish Immunol. 2025. PMID: 40639701
-
Metal-amino acid complexes (Zn, Se, Cu, Fe, and Mn) enhance immune response, antioxidant capacity, liver function enzymes, and expression of cytokine genes in Nile Tilapia reared under field conditions.J Aquat Anim Health. 2023 Dec;35(4):248-262. doi: 10.1002/aah.10194. Epub 2023 Nov 16. J Aquat Anim Health. 2023. PMID: 37501584
-
Efficacy of dietary Ceratonia silique and Zingiber offcinale on the immune-antioxidant-signaling pathways, growth, physiological response, and ammonia resistance in Oreochromis niloticus reared under unchanged water.Fish Physiol Biochem. 2025 May 22;51(3):100. doi: 10.1007/s10695-025-01496-w. Fish Physiol Biochem. 2025. PMID: 40402292 Free PMC article.
-
Mitigation of cold stress in Nile tilapia (Oreochromis niloticus) through dietary lipids supplementation: a preliminary network meta-analysis.Fish Physiol Biochem. 2024 Feb;50(1):209-223. doi: 10.1007/s10695-023-01217-1. Epub 2023 Jul 15. Fish Physiol Biochem. 2024. PMID: 37453980 Review.
-
A Blend of natural phytobiotics enhances growth performance, feed efficiency, and the immuno-health status of fingerlings of Nile tilapia (Oreochromis niloticus).Open Vet J. 2025 Feb;15(2):746-764. doi: 10.5455/OVJ.2025.v15.i2.24. Epub 2025 Feb 28. Open Vet J. 2025. PMID: 40201808 Free PMC article. Review.
References
-
- FAO. FAO yearbook of fishery and aquaculture statistics. Rome. (2024).
-
- FAO. Food and Agriculture Organization of the United Nations: The state of world fisheries and aquaculture. Rome, Italy. Retrieved from (2022). https://www.fao.org/3/cc0461en/cc0461en.pdf.
-
- El-Ouny, Y. M. et al. Effect of fishmeal replacement with dried red wigglers (Eisenia fetida) worm meal on growth and feed utilization, production efficiency, and serum biochemistry in nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture Rep.29, 101518 (2023).
-
- Abdel-Latif, H. M. R. et al. Growth Performance, Antioxidant Activities, and Immunological Responses of hapa-reared Thinlip Mullet (Liza ramada) Juveniles Fed on Diets Supplemented with spirulina (Arthrospira platensis)130p. 359–367 (Fish & Shellfish Immunology, 2022). - PubMed
-
- Khalil, H. S. & Nasr-Allah, A. Comparative study on the effect of raceway and In-Pond raceway systems on different nile tilapia, Oreochromis niloticus strains fed diets replacing soybean meal by poultry byproduct meal on: water quality, growth performance and production efficiency. Aquacult. Int.33 (4), 258 (2025).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

