AAV for ovarian cancer gene therapy
- PMID: 40542144
- PMCID: PMC12353829
- DOI: 10.1038/s41417-025-00926-4
AAV for ovarian cancer gene therapy
Abstract
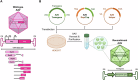
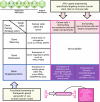
Recent advancements in ovarian cancer treatment, particularly with PARP inhibitors, have markedly enhanced the recurrence-free interval, shifting the treatment paradigm and increasing treatment success in patients with BRCA mutations or HRD (homologous recombination deficiency). However, a significant proportion of cases experience relapse, resulting in poorer long-term survival rates when compared to other female cancers, such as breast cancer. This review explores the potential of adeno-associated virus (AAV) vectors for gene therapy in ovarian cancer and examines rational gene therapy strategies by categorizing them based on target cells and target genes to determine the most effective approach for ovarian cancer treatment. Specifically, it examines strategies such as anti-angiogenesis and immune modulation, highlighting the strategy of gene supplementation to hinder ovarian cancer progression. Innovations in AAV capsid design now allow for targeted delivery, focusing on ovarian cancer stem cells (CSCs) identified by specific markers. Additionally, leveraging DNA sequencing technologies enhances the identification and incorporation of therapeutic genes into AAV vectors, promising new avenues for ovarian cancer gene therapy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
The Molecular Mechanisms of Actions, Effects, and Clinical Implications of PARP Inhibitors in Epithelial Ovarian Cancers: A Systematic Review.Int J Mol Sci. 2022 Jul 23;23(15):8125. doi: 10.3390/ijms23158125. Int J Mol Sci. 2022. PMID: 35897700 Free PMC article.
-
Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer.Cochrane Database Syst Rev. 2022 Feb 16;2(2):CD007929. doi: 10.1002/14651858.CD007929.pub4. Cochrane Database Syst Rev. 2022. PMID: 35170751 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Immune responses to central nervous system directed adeno-associated virus gene therapy: Does direct CNS delivery make a difference?Neurotherapeutics. 2024 Jul;21(4):e00435. doi: 10.1016/j.neurot.2024.e00435. Epub 2024 Aug 23. Neurotherapeutics. 2024. PMID: 39180957 Free PMC article. Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

