Delphinidin attenuates cognitive deficits and pathology of Alzheimer's disease by preventing microglial senescence via AMPK/SIRT1 pathway
- PMID: 40542425
- PMCID: PMC12180243
- DOI: 10.1186/s13195-025-01783-x
Delphinidin attenuates cognitive deficits and pathology of Alzheimer's disease by preventing microglial senescence via AMPK/SIRT1 pathway
Abstract
Background: Emerging evidence suggests that senescent microglia play a role in β-amyloid (Aβ) pathology and neuroinflammation in Alzheimer's disease (AD). Targeting senescent cells with naturally derived compounds exhibiting minimal cytotoxicity represents a promising therapeutic strategy.
Objectives: This study aimed to investigate whether delphinidin, a naturally occurring anthocyanin, can alleviate AD-related pathologies by mitigating microglial senescence and to elucidate the underlying molecular mechanisms.
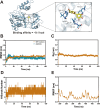
Methods: We employed APP/PS1 mice, naturally aged mice, and an in vitro model using Aβ42-induced senescent BV2 microglia. Delphinidin's effects were evaluated through assessments of cognitive function, synaptic integrity (synapse loss), Aβ plaque burden, senescent microglia gene signatures, and cellular senescence markers (including senescence-associated β-galactosidase activity, SASP factor expression, oxidative stress, and cyclin p21/p16 levels). Mechanistic studies involved analyzing the AMPK/SIRT1 signaling pathway, testing direct delphinidin-SIRT1 interaction, and using the AMPK inhibitor Compound C.
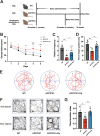
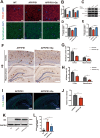
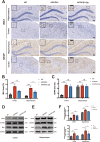
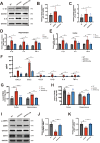
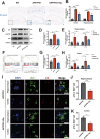
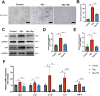
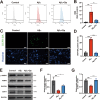
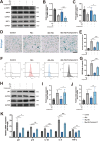
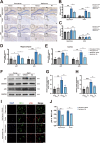
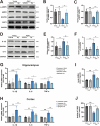
Results: Delphinidin treatment significantly alleviated cognitive deficits, synapse loss, Aβ peptides plaques of APP/PS1 mice via downregulated senescent microglia gene signature, prevented cell senescence, including senescence-associated β-galactosidase activity, senescence-associated secretory phenotype (SASP), oxidative stress, cyclin p21 and p16. And delphinidin treatment also prevented microglial senescence in naturally aged mice. In vitro, delphinidin treatment attenuated cell senescence induced by Aβ42 in BV2 microglia cells. Further research indicated that delphinidin treatment enhanced the AMPK/SIRT1 signaling pathway. Additionally, delphinidin was found to directly interact with SIRT1. It's noteworthy that AMPK inhibitor Compound C inversed the protective effect of delphinidin against microglial senescence.
Conclusion: Our study reveals for the first time that delphinidin effectively improved cognitive deficits, alleviated synapse loss and Aβ pathology in APP/PS1 mice by mitigating microglial senescence. These findings highlight delphinidin as a promising natural anti-aging agent against the development of aging and age-related diseases.
Keywords: AMPK; Aging; Alzheimer's disease; Microglial senescence; Neuroinflammation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Approval of the study protocol was obtained from the Experimental Animal Ethics Committee of Fudan University (License No. 2022110017S). All animal experimental were conducted in compliance with the guidelines established by the Animal Research: Reporting of In Vivo Experiments (ARRIVE). Competing interests: The authors declare no competing interests.
Figures

References
-
- van der Kant R, Goldstein LSB, Ossenkoppele R. Amyloid-beta-independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci. 2020;21(1):21–35. - PubMed
-
- Garwood CJ, Simpson JE, Al Mashhadi S, Axe C, Wilson S, Heath PR, et al. DNA damage response and senescence in endothelial cells of human cerebral cortex and relation to Alzheimer’s neuropathology progression: a population-based study in the Medical Research Council Cognitive Function and Ageing Study (MRC-CFAS) cohort. Neuropathol Appl Neurobiol. 2014;40(7):802–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

