Regorafenib plus modified gemcitabine-oxaliplatin in patients with advanced biliary tract cancer. The randomized phase Ib/II BREGO study
- PMID: 40542585
- PMCID: PMC12204757
- DOI: 10.1093/oncolo/oyaf080
Regorafenib plus modified gemcitabine-oxaliplatin in patients with advanced biliary tract cancer. The randomized phase Ib/II BREGO study
Abstract
Background: New therapeutic options are needed for biliary tract cancer (BTC). Regorafenib, a multikinase inhibitor, shows promise in refractory digestive cancers and may be beneficial with conventional chemotherapy for BTC.
Patients and methods: The BREGO study evaluated regorafenib with modified gemcitabine-oxaliplatin (mGEMOX) in advanced or metastatic BTC. Phase I determined the recommended dose (RP2D) of regorafenib (80, 120 or 160 mg, days 1-14) combined with mGEMOX (gemcitabine 900 mg.m-2 IV, 30 min, followed by oxaliplatin 80mg.m-2 IV, 120 min, days 1 and 8). Phase II randomized (1:2) patients to mGEMOX alone (arm A) or mGEMOX + regorafenib (arm B, RP2D, days 1-14), assessing efficacy and safety, with the primary outcome being progression-free survival (PFS). Metabolic tumor features and response were also assessed.
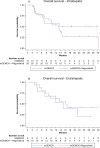
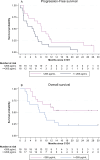
Results: In phase Ib, 22 patients were enrolled; in phase II, 66 patients (arm A, n = 24; arm B, n = 42). Four dose-limiting toxicities were observed, but no maximum tolerated dose was reached. The RP2D was 160 mg.d-1. Median PFS (7.2 vs7.8 months; P = .825) and overall survival (15.1 vs 13.5 months; P = .356) were similar between arms. However, posttreatment 18F-FDG tumor uptake (SULpeak) significantly correlated with PFS (P = .001) and OS (P = .016). Baseline plasma stanniocalcin 1 levels < 265 pg.mL-1 were associated with longer PFS (P = .030) and OS (P = .060) in both arms.
Conclusions: Combining regorafenib and mGEMOX is feasible as first-line treatment for BTC but did not increase PFS as expected in the phase II cohort. Identifying new biomarkers can help target patients with advanced BTCs who may benefit from regorafenib-associated therapy.
Trial registration number: ClinicalTrials.gov, NCT02386397.
Keywords: biliary tract cancer; modified GEMOX; regorafenib; stanniocalcin 1.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
J.-F.B. received honoraria for speaking or consulting roles from Bayer, AstraZeneca, MSD, Servier, Incyte, Jazz Pharmaceutical, Tahio. M.B. received speaker fees from Bayer, MSD, Sirtex Medical, and Roche, and advisory board fees from Bayer, MSD, Sirtex Medical, Eisai, AstraZeneca, Ipsen, Servier, Taiho, and BMS. Y.T. received board fees and speakers invitations from Bayer. C.B. received honoraria from Amgen, Bayer, Beigine, Biocartis, Bristol Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Merck, Pfizer, Pierre Fabre, Roche, Sanofi, SeqOne, Servier, and Takeda. L.M. received personal fees from Servier, Amgen, Sanofi, Astrazeneca, Pfizer, Merck, Amgen, Bayer Ipesen, Lilly, and Roche. He also received honoraria from Sanofi, Eisai, Servier, Ipsen, Amgen, and Merck and for consulting role from Sanofi, Sandoz, Kephren, Bayer, Merck, Ipsen, Erytech, Lilly, and Servier. He also received research grants from Sanofi, Merck, and Chugai. T.M. received honoraria for speaking or consulting roles from Servier, Pierre Fabre, Merck Serono, AAA, Sanofi, Galapagos, and research grants from Amgen, and Roche. He also received travel grants from Pierre Fabre, Merck Serono, and Sanofi. E.A. received honoraria for speaking or consulting roles from Roche, Novartis, MSD, AstraZeneca, Bayer, Ipsen, Boston, Incyte, Servier, and AAA. All other authors have declared no conflicts of interest.
Figures

Similar articles
-
Gemcitabine-based chemotherapy for advanced biliary tract carcinomas.Cochrane Database Syst Rev. 2018 Apr 6;4(4):CD011746. doi: 10.1002/14651858.CD011746.pub2. Cochrane Database Syst Rev. 2018. PMID: 29624208 Free PMC article.
-
Atezolizumab Plus Chemotherapy With or Without Bevacizumab in Advanced Biliary Tract Cancer: Clinical and Biomarker Data From the Randomized Phase II IMbrave151 Trial.J Clin Oncol. 2025 Feb 10;43(5):545-557. doi: 10.1200/JCO.24.00337. Epub 2024 Oct 18. J Clin Oncol. 2025. PMID: 39423355 Free PMC article. Clinical Trial.
-
A Phase II Trial of Pemetrexed Plus Oxaliplatin in Patients With Advanced Biliary Tract Cancer After Failure of Gemcitabine Plus Cisplatin-based Chemotherapy.Anticancer Res. 2025 Sep;45(9):3983-3991. doi: 10.21873/anticanres.17756. Anticancer Res. 2025. PMID: 40877003 Clinical Trial.
-
Health-related quality of life in participants with advanced biliary tract cancer from the randomized phase III KEYNOTE-966 study.J Hepatol. 2025 Sep;83(3):692-700. doi: 10.1016/j.jhep.2025.03.019. Epub 2025 Mar 27. J Hepatol. 2025. PMID: 40154623 Clinical Trial.
-
Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: a systematic review.Cancer Med. 2014 Dec;3(6):1502-11. doi: 10.1002/cam4.299. Epub 2014 Aug 11. Cancer Med. 2014. PMID: 25111859 Free PMC article.
References
-
- Valle JW, Kelley RK, Nervi B, Oh D-Y, Zhu AX.. Biliary tract cancer. Lancet. 2021;397:428-444. https://doi.org/ 10.1016/S0140-6736(21)00153-7 - DOI - PubMed
-
- Lepage C, Cottet V, Chauvenet M, et al. Trends in the incidence and management of biliary tract cancer: a French population-based study. J Hepatol. 2011;54:306-310. https://doi.org/ 10.1016/j.jhep.2010.06.039 - DOI - PubMed
-
- Valle J, Wasan H, Palmer DH, et al. ; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. https://doi.org/ 10.1056/NEJMoa0908721 - DOI - PubMed
-
- Lamarca A, Palmer DH, Wasan HS, et al. ; Advanced Biliary Cancer Working Group. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690-701. https://doi.org/ 10.1016/S1470-2045(21)00027-9 - DOI - PMC - PubMed
-
- Boilève A, Hilmi M, Delaye M, Tijeras-Raballand A, Neuzillet C.. Biomarkers in hepatobiliary cancers: what is useful in clinical practice? Cancers (Basel). 2021;13:2708. https://doi.org/ 10.3390/cancers13112708 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

