Paraventricular Nucleus of the Thalamus Neurons That Project to the Nucleus Accumbens Show Enhanced c-Fos Expression During Early-Stage Cue-Reward Associative Learning in Male Rats
- PMID: 40542605
- PMCID: PMC12181800
- DOI: 10.1111/ejn.70168
Paraventricular Nucleus of the Thalamus Neurons That Project to the Nucleus Accumbens Show Enhanced c-Fos Expression During Early-Stage Cue-Reward Associative Learning in Male Rats
Abstract
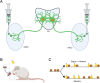
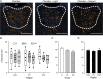
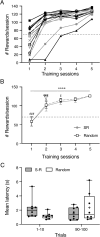
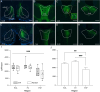
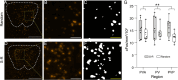
The paraventricular nucleus of the thalamus (PVT) is a central node in brain networks controlling motivated behaviors. It processes inputs from cerebral cortex, brainstem, and hypothalamus and has efferents that project to a wide range of structures, including the nucleus accumbens (nAcc). It is known that PVT neurons projecting to the nAcc show c-Fos activation in response to reward-related cues, in well-trained animals. We previously found that c-Fos expression is also increased early in the conditioning process, during the first session of learning a new cue-reward association in rats, but neurons with projections to nAcc were not identified in that study. Here, we tested the hypothesis that nAcc-projecting PVT neurons would show this enhanced c-Fos expression following first exposure to the association of a visual cue with a subsequent food reward. Male rats were stereotaxically injected in the nAcc with a retrogradely transported adeno-associated virus construct leading to green fluorescent protein (GFP) expression in cell bodies of afferents from PVT. Following a single session of cue-reward training, processing of the brains with dual immunohistochemistry for c-Fos and GFP showed significantly higher density of double labelled neurons in the conditioned group, compared to controls in which the same number of cues and rewards were delivered at random intervals with respect to each other. Such activation of immediate early gene expression in PVT to nAcc projecting neurons very early in paired associative reward learning may have a role in modulating plasticity in the nAcc.
Keywords: immediate early gene; midline thalamus; retrograde adeno‐associated viral vector.
© 2025 The Author(s). European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
A food-predictive cue attributed with incentive salience engages subcortical afferents and efferents of the paraventricular nucleus of the thalamus.Neuroscience. 2017 Jan 6;340:135-152. doi: 10.1016/j.neuroscience.2016.10.043. Epub 2016 Oct 25. Neuroscience. 2017. PMID: 27793779 Free PMC article.
-
Limbic-motor integration by neural excitations and inhibitions in the nucleus accumbens.J Neurophysiol. 2017 Nov 1;118(5):2549-2567. doi: 10.1152/jn.00465.2017. Epub 2017 Aug 9. J Neurophysiol. 2017. PMID: 28794196 Free PMC article.
-
Tolerance in Thalamic Paraventricular Nucleus Neurons Following Chronic Treatment of Animals with Morphine.eNeuro. 2025 Jun 11;12(6):ENEURO.0249-24.2025. doi: 10.1523/ENEURO.0249-24.2025. Print 2025 Jun. eNeuro. 2025. PMID: 40456612 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Jul 06;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. PMID: 17253490 Updated.
References
-
- Choi, D. L. , Davis J. F., Fitzgerald M. E., and Benoit S. C.. 2010. “The Role of Orexin‐A in Food Motivation, Reward‐Based Feeding Behavior and Food‐Induced Neuronal Activation in Rats.” Neuroscience 167: 11–20. - PubMed
-
- Conte, W. L. , Kamishina H., and Reep R. L.. 2009. “The Efficacy of the Fluorescent Conjugates of Cholera Toxin Subunit B for Multiple Retrograde Tract Tracing in the Central Nervous System.” Brain Structure & Function 213: 367–373. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

