Mitochondria-Homing Drug Mitochonic Acid 5 Improves Barth Syndrome Myopathy in a Human-Induced Pluripotent Stem Cell Model and Barth Syndrome Drosophila Model
- PMID: 40542649
- PMCID: PMC12181814
- DOI: 10.1096/fj.202401856RRR
Mitochondria-Homing Drug Mitochonic Acid 5 Improves Barth Syndrome Myopathy in a Human-Induced Pluripotent Stem Cell Model and Barth Syndrome Drosophila Model
Abstract
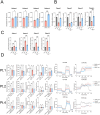
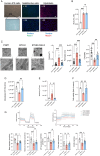
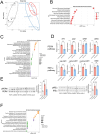
Barth syndrome (BTHS) is a rare disease caused by mutations in the tafazzin gene that affects the heart and muscles; however, to date, no clinically effective drugs are available. In BTHS, mitochondrial function is reduced owing to changes in cardiolipin metabolism. We developed mitochonic acid 5 (MA-5), a small-molecule compound that increases ATP levels, improves mitochondrial dynamics, and is effective in treating mitochondrial and muscle diseases. Therefore, this study examined the effectiveness of MA-5 in treating BTHS. The mitochondrial functions of four isolated BTHS skin fibroblasts were examined. Human BTHS induced pluripotent stem cell (iPSC) were differentiated into myoblasts and cardiolipin metabolism and mitochondrial functions were analyzed. RNA-seq was performed to clarify the metabolic changes. Using a Drosophila melanogaster model of BTHS, the effects of MA-5 on motor performance and cardiac phenotype were examined. MA-5 improved mitochondrial function and reduced cell death due to oxidative stress in skin fibroblasts of patients with BTHS. MA-5 promoted ATP production and reduced oxidative stress in human BTHS iPS cell-derived myoblasts. RNA-seq analysis revealed that MA-5 alleviated endoplasmic reticulum stress in BTHS cells. Administration of MA-5 to BTHS Drosophila improved locomotor ability and tachycardia observed in patients with BTHS. Protein interaction analyses suggested colocalization of ATPase and the MA-5-binding protein mitofilin. These data suggested that MA-5 improves BTHS dysfunction and may serve as a novel therapeutic agent for BTHS.
Keywords: Drosophila; ATP; Barth syndrome; cardiolipin; iPS; mitochondria.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors have nothing to report.
The authors declare no conflicts of interest.
Figures



Similar articles
-
Allogenic mitochondria transfer improves cardiac function in iPS-cell-differentiated cardiomyocytes of a patient with Barth syndrome.Exp Mol Med. 2025 Jun;57(6):1260-1271. doi: 10.1038/s12276-025-01472-7. Epub 2025 Jun 24. Exp Mol Med. 2025. PMID: 40555742 Free PMC article.
-
Granulopoietic Dysregulation in a Patient-Tailored Mouse Model of Barth Syndrome.Stem Cell Rev Rep. 2025 Oct;21(7):2170-2187. doi: 10.1007/s12015-025-10945-1. Epub 2025 Aug 5. Stem Cell Rev Rep. 2025. PMID: 40762880 Free PMC article.
-
Stem cell models of TAFAZZIN deficiency reveal novel tissue-specific pathologies in Barth syndrome.Hum Mol Genet. 2025 Jan 23;34(1):101-115. doi: 10.1093/hmg/ddae152. Hum Mol Genet. 2025. PMID: 39535077
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Barth P. G., Valianpour F., Bowen V. M., et al., “X‐Linked Cardioskeletal Myopathy and Neutropenia (Barth Syndrome): An Update,” American Journal of Medical Genetics, Part A 126A (2004): 349–354. - PubMed
-
- Kang S. L., Forsey J., Dudley D., Steward C. G., and Tsai‐Goodman B., “Clinical Characteristics and Outcomes of Cardiomyopathy in Barth Syndrome: The UK Experience,” Pediatric Cardiology 37 (2016): 167–176. - PubMed
MeSH terms
Grants and funding
- 21H02932/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20ek0210133h0001/Japan Agency for Medical Research and Development (AMED)
- 20ak0101127h0001/Japan Agency for Medical Research and Development (AMED)
- JP22zf0127001/Japan Agency for Medical Research and Development (AMED)
- 24zf0127001h0004/Japan Agency for Medical Research and Development (AMED)
LinkOut - more resources
Full Text Sources
Medical

