Implementation of guidelines for intravenous iron therapy in heart failure patients
- PMID: 40542671
- PMCID: PMC12450754
- DOI: 10.1002/ehf2.15340
Implementation of guidelines for intravenous iron therapy in heart failure patients
Abstract
Aims: We aim to evaluate the implementation of the 2021 ESC Guidelines regarding screening and treatment of iron deficiency in patients with heart failure (HF) at Karolinska University Hospital.
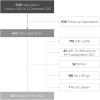
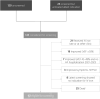
Methods and results: Patients with left ventricular ejection fraction (LVEF) < 50% and New York Heart Association (NYHA) class II-IV who visited Karolinska University Hospital in 2021 were identified through the registry SwedeHF. Data on patient characteristics, screening for iron deficiency, administration of ferric carboxymaltose (FCM) and HF medication were extracted from patients' health records. There were 261 patients registered whereof 141 (54%) were screened. Screened patients had lower LVEF [33% vs. 36% (P = 0.033)], higher N terminal pro brain natriuretic peptide levels [2880 ng/L vs. 1960 ng/L (P = 0.040)], higher prevalence of diabetes [31.9% vs. 20.0% (P = 0.030)] and were more often prescribed sodium-glucose co-transporter 2 inhibitors (SGLT2i) (49.6% vs. 25.8%) and loop diuretics [76.6% vs. 56.7% (both P < 0.001] compared with unscreened patients. Patients treated with intravenous iron had a higher NYHA class than untreated patients with an indication for treatment (P = 0.038).
Conclusions: In patients with HFrEF and HFmrEF, 54% were screened for iron deficiency. Screened patients had more advanced HF disease, possibly due to increased medical attention and higher compliance to guideline recommended treatment. We demonstrate that national quality registries can track outcomes and effectively enhance patient care.
Keywords: Guidelines; Heart failure; Implementation science; Iron deficiency.
© 2025 The Author(s). ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
Dr. Savarese reports grants and personal fees from Vifor, Boehringer Ingelheim, AstraZeneca, Servier, Novartis, Cytokinetics, Pharmacosmos, and personal fees from Roche, Abbott, Edwards Lifescience, Medtronic, TEVA, INTAS and grants from Boston Scientific, Merck, Bayer, all outside the submitted work. Camilla Hage reports consulting fees from Novartis, Roche Diagnostics and AnaCardio, research grants from Bayer and speaker and honoraria from AstraZeneca and Novartis.
Figures

References
-
- Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross‐sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J 2002;23:1867‐1876. doi: 10.1053/euhj.2002.3255 - DOI - PubMed
-
- Socialstyrelsen . DRG‐statistik 2019 [Internet]. [cited 2023 Feb 12]. Report No.: 2020–11–7042. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint‐dokument/artikelk...: Socialstyrelsen.; 2023. Accessed 01 January 2021
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

