FLOT chemotherapy treatment affects adipocyte's lipid metabolism: an in vitro study
- PMID: 40542676
- PMCID: PMC12445512
- DOI: 10.1080/21623945.2025.2518285
FLOT chemotherapy treatment affects adipocyte's lipid metabolism: an in vitro study
Abstract
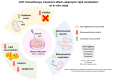
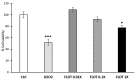
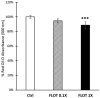
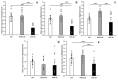
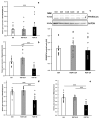
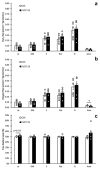
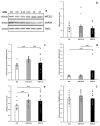
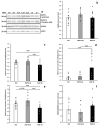
Cachexia is a complex syndrome that is often associated with cancer. Chemotherapy, one of the main cancer treatments, worsens weight loss in cancer-induced cachexia. In this context, it is thought that fat loss precedes muscle loss, and that alterations in adipose tissue are associated with tumours. However, the effect of cancer treatment on adipose tissue is not well understood. This study aimed to evaluate the impact of chemotherapy alone on mature 3T3-L1 adipocytes to identify the mechanisms contributing to adipose tissue alteration. The murine cell line 3T3-L1, a model of mature adipocytes, was used in this study. After differentiation, cells were treated for 48 h with a chemotherapy cocktail called FLOT composed of 5-fluorouracil, leucovorin, oxaliplatin and docetaxel at two concentrations (FLOT 1X and 0.1X). The control group was treated with the vehicle of the chemotherapy cocktail. Viability, mitochondrial function and dynamics, lipid metabolism, and cellular stress were also evaluated. FLOT 1X chemotherapy significantly reduced viability of mature 3T3-L1 cells and inhibited lipid accumulation. Interestingly, while FLOT 1X treatment downregulated lipogenesis markers, FLOT 0.1X treatment upregulated some of them. Although, the treatment showed no effect on mitochondrial respiration or density, it significantly increased expression of oxidative stress and inflammation markers in adipocytes.This in vitro study provides the first evidence of FLOT chemotherapy's direct effects on healthy mature adipocytes. The results demonstrate significant treatment-induced reductions in cell viability along with dysregulation of both lipogenic and lipolytic pathways. These findings elucidate previously unrecognized mechanisms underlying adipose tissue dysfunction in cancer cachexia.
Keywords: FLOT; adipocytes; high resolution respirometry; lipid metabolism; mouse; reactive oxygen species.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Al-Batran S-E, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–1957. doi: 10.1016/S0140-6736(18)32557-1 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
