Blocking leukotriene receptors improve experimentally induced gastric ulcers in rats by inhibiting inflammation and apoptosis
- PMID: 40542712
- PMCID: PMC12182629
- DOI: 10.1177/03946320251351083
Blocking leukotriene receptors improve experimentally induced gastric ulcers in rats by inhibiting inflammation and apoptosis
Abstract
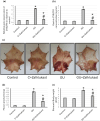
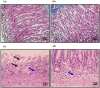
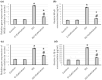
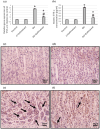
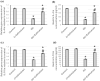
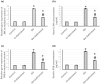
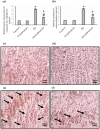
To investigate whether obstructing the cysteinyl leukotriene receptor-1 (CYSLTR1) with zafirlukast diminishes experimentally induced gastric ulcer (GU) in rats by modulating inflammation and apoptosis. Gastric ulcers affect approximately 10% of the global population and can lead to serious complications such as gastrointestinal perforation and bleeding. Leukotrienes are proinflammatory compounds, and cysteinyl leukotrienes, such as LTC4, LTD4, and LTE4, that are potent proinflammatory mediators. Rats were orally administered a single oral dose of 80 mg/kg of indomethacin to induce GU. The rats were administered an oral dose of 20 mg/kg Zafirlukast. Gastric tissues were collected for macrostructural and microstructural analyses. A portion of gastric tissue was used to assess the genetic expression and protein levels of CYSLTR1, NFκB, TNF-α, IL-1β/4/10, JNK, PKB, and caspase-3. The gastric sections were subjected to hematoxylin/eosin and Masson trichrome staining and immunohistochemical staining with anti-TNF-α and anti-caspase-3 antibodies. Zafirlukast blocked the expression of CYSLTR1. Analysis of micro-images of GU rats revealed damage to surface cells and glandular epithelial cells caused by inflammatory cell infiltration, which was mitigated by Zafirlukast. Additionally, Zafirlukast treatment significantly reduced NFκB, TNF-α, IL-1β, JNK, PKB, and caspase-3 while increasing IL-4 and IL-10. Zafirlukast successfully reduced experimentally induced gastric ulcers in rats. Its mechanism of action includes inhibition of CYSLTR1, diminishing the inflammatory pathway. This is demonstrated by a decrease in the levels of NFκB, TNF-α, and IL-1β, along with an increase in the levels of IL-4 and IL-10. Additionally, Zafirlukast exerted anti-apoptotic effects by downregulating the expression of JNK, PKB, and caspase-3.
Keywords: Jun N-terminal kinase (JNK); cysteinyl leukotriene receptor 1 (CYSLTR1); nuclear factor (NF)κB; protein kinase B (PKB); tumor necrosis factor-α (TNF-α).
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Effectiveness of the fruit of Rosa odorata sweet var. gigantea (Coll. et Hemsl.) Rehd. et Wils in the protection and the healing of ethanol-induced rat gastric mucosa ulcer based on Nrf2/NF-κB pathway regulation.J Ethnopharmacol. 2022 Jan 10;282:114626. doi: 10.1016/j.jep.2021.114626. Epub 2021 Sep 10. J Ethnopharmacol. 2022. PMID: 34517064
-
Explore dual anti-inflammatory and cell protective mechanisms the mechanism of Jianwei Yuyang tablet in the treatment of alcohol-induced gastric ulcers via bioinformatics and experimental validation.Phytomedicine. 2025 Aug;144:156936. doi: 10.1016/j.phymed.2025.156936. Epub 2025 Jun 1. Phytomedicine. 2025. PMID: 40482617
-
In Vivo Evidence for the Preventive Role of Vaccinium macrocarpon Aiton in Indomethacin-Induced Gastric Ulcer: Focusing on Antioxidant, Anti-Inflammatory and Anti-Apoptotic Mechanisms.Vet Med Sci. 2025 Jan;11(1):e70048. doi: 10.1002/vms3.70048. Vet Med Sci. 2025. PMID: 39745481 Free PMC article.
-
Gastroprotective Role of Fruit Extracts in Gastric Damage Induced by Non-Steroidal Anti-Inflammatory Drugs: A Systematic Review.J Med Food. 2023 Nov;26(11):777-798. doi: 10.1089/jmf.2023.0005. Epub 2023 Oct 30. J Med Food. 2023. PMID: 37902784
-
Addition of anti-leukotriene agents to inhaled corticosteroids for adults and adolescents with persistent asthma.Cochrane Database Syst Rev. 2017 Mar 16;3(3):CD010347. doi: 10.1002/14651858.CD010347.pub2. Cochrane Database Syst Rev. 2017. PMID: 28301050 Free PMC article.
References
-
- Tanigawa T, Pai R, Arakawa T, et al. TGF-beta signaling pathway: Its role in gastrointestinal pathophysiology and modulation of ulcer healing. J Physiol Pharmacol 2005; 56: 3–13. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

