Efficacy of Lenvatinib Versus Sorafenib in the Treatment of Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
- PMID: 40542755
- PMCID: PMC12374529
- DOI: 10.31557/APJCP.2025.26.6.1943
Efficacy of Lenvatinib Versus Sorafenib in the Treatment of Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Abstract
Introduction: Hepatocellular carcinoma (HCC) was the third leading cause of cancer-related deaths in the world. Current global treatment recommendations suggest lenvatinib and sorafenib have been approved to treat unresectable HCC. Studies comparing lenvatinib versus sorafenib for unresectable HCC have shown conflicting results and no structured review has yet evaluated its efficacy and safety. This article aims to estimate the efficacy of lenvatinib and sorafenib in patients with unresectable HCC.
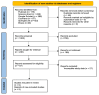
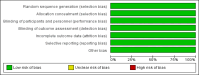
Methods: This research was conducted using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) strategy. Literature searches were conducted through PubMed, ScienceDirect, Google Scholar, Cochrane Library, SpringerLink, and Ebsco. After quality assessment using the Newcastle-Ottawa Scale (NOS) and Cochrane Risk-of-bias, also data extraction, Review Manager 5.4 and RStudio 2024.04.1 software were used for analysis of overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR).
Results: A total of 9 studies were included, comprising 3,821 samples. All studies were retrospective studies. Our meta-analysis showed that OS and PFS in patients receiving lenvatinib were significantly better than patients receiving sorafenib with a protective hazard ratio (HR) of 0.70 (95%CI: 0.57-0.87, p=0.001) and 0.65 (95%CI: 0.54-0.78; p < 0.00001) respectively. Moreover, in the viral patients group, lenvatinib showed similar OS compared with sorafenib (HR=1.02; 95%CI: 0.77-1.36, p=0.87). Lenvatinib exhibited better ORR (OR = 7.87; 95%CI: 2.02-30.75; p = 0.003) and DCR (OR = 1.99; 95%CI: 1.53-2.60; p < 0.00001) compared with sorafenib.
Conclusion: Lenvatinib provided significant benefits in OS, PFS, ORR, and DCR compared to sorafenib in patients with unresectable HCC.
Keywords: Hepatocellular carcinoma; Lenvatinib; sorafenib.
Conflict of interest statement
All authors declare there was no conflict of interest regarding this study.
Figures

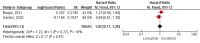


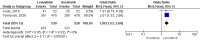
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

