Five-year safety and growth response of long-acting PEGylated recombinant human growth hormone in children with growth hormone deficiency-data from CGLS database
- PMID: 40542826
- PMCID: PMC12182472
- DOI: 10.1007/s00431-025-06268-5
Five-year safety and growth response of long-acting PEGylated recombinant human growth hormone in children with growth hormone deficiency-data from CGLS database
Abstract
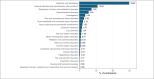
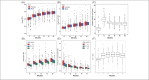
Growth hormone deficiency (GHD) is an endocrine disorder characterized by insufficient production of growth hormone (GH). PEGylated recombinant human growth hormone (PEG-rhGH; Jintrolong®, GeneScience Pharmaceuticals Co., Ltd.) is the only long-acting GH approved in China for treating paediatric GHD (PGHD). Long-term Efficacy and Safety Evaluation of Growth Hormone in Children in China (CGLS) is a large, surveillance registry database of participants with short stature treated with PEG-rhGH or rhGH in a real-world setting. In this study, we evaluated the safety profile and five-year growth response of PEG-rhGH based on the data from the CGLS database in participants with PGHD in China. In this real-world registry-based observational study, a total of 1,207 participants were included in the safety analysis set. Of these, 339 participants who had received PEG-rhGH continuously for five years were also included in the efficacy analysis. Key outcomes assessed comprised adverse events (AEs), serious AEs (SAEs), and height gain. The safety assessment indicated that 563 participants exhibited 1328 AEs with an incidence rate of 46.6%. Furthermore, SAEs occurred in 1.0% of participants (n = 12), with none of them associated with PEG-rhGH treatment. A significant increase in mean change in height-SD score (∆Ht SDS) was observed during the treatment period, with a mean ∆Ht SDS of 2.1 ± 0.9 in five years. Subgroup analysis showed that the younger participants exhibited a more favourable response to therapy.
Conclusion: CGLS data showed that five-year PEG-rhGH treatment in children with PGHD was associated with a favourable safety profile and sustained height gain.
What is known: • Several long-acting growth hormones (LAGH) have been approved for use in PGHD. PEGylated recombinant growth hormone (PEG-rhGH) is the only LAGH marketed in China, with its three-year efficacy and safety have been reported.
What is new: • Data from the CGLS database confirms that PEG-rhGh has an acceptable safety profile over five years, with significant improvement in height. Importantly, the data indicate that initiating the treatment earlier yields better outcomes.
Keywords: CGLS database; Growth hormone; Growth response; PEG-rhGH; Safety.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures
Similar articles
-
Real-Life Growth Hormone Treatment Patterns in Children from China: A Report from Two Databases.Adv Ther. 2025 Jul;42(7):3562-3575. doi: 10.1007/s12325-025-03204-9. Epub 2025 May 29. Adv Ther. 2025. PMID: 40439954 Free PMC article.
-
Comparison between long-acting pegylated and daily recombinant human growth hormone for pediatric growth hormone deficiency a systematic review.Sci Rep. 2025 Jul 23;15(1):26746. doi: 10.1038/s41598-025-10613-x. Sci Rep. 2025. PMID: 40702077 Free PMC article.
-
Recombinant human growth hormone for the treatment of growth disorders in children: a systematic review and economic evaluation.Health Technol Assess. 2010 Sep;14(42):1-209, iii-iv. doi: 10.3310/hta14420. Health Technol Assess. 2010. PMID: 20849734
-
A Novel Y-Shaped Pegylated Recombinant Human Growth Hormone for Children With Growth Hormone Deficiency.J Clin Endocrinol Metab. 2025 Jul 15;110(8):e2605-e2613. doi: 10.1210/clinem/dgae816. J Clin Endocrinol Metab. 2025. PMID: 39607675 Free PMC article. Clinical Trial.
-
Growth hormone for children with chronic kidney disease.Cochrane Database Syst Rev. 2012 Feb 15;2012(2):CD003264. doi: 10.1002/14651858.CD003264.pub3. Cochrane Database Syst Rev. 2012. PMID: 22336787 Free PMC article.
References
-
- Hage C, Gan H-W, Ibba A et al (2021) Advances in differential diagnosis and management of growth hormone deficiency in children. Nat Rev Endocrinol 17:608–624. 10.1038/s41574-021-00539-5 - PubMed
-
- Chinoy A, Murray PG (2016) Diagnosis of growth hormone deficiency in the paediatric and transitional age. Best Pract Res Clin Endocrinol Metab 30:737–747. 10.1016/j.beem.2016.11.002 - PubMed
-
- Bao XL, Shi YF, Du YC et al (1992) Prevalence of growth hormone deficiency of children in Beijing. Chin Med J (Engl) 105:401–405 - PubMed
-
- Ranke MB, Lindberg A, Albertsson-Wikland K et al (2005) Increased Response, But Lower Responsiveness, to Growth Hormone (GH) in Very Young Children (Aged 0–3 Years) with Idiopathic GH Deficiency: Analysis of Data from KIGS. J Clin Endocrinol Metab 90:1966–1971. 10.1210/jc.2004-1051 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

