Post-traumatic hydrocephalus after decompressive craniectomy: a multidimensional analysis of clinical, radiological, and surgical risk factors
- PMID: 40542880
- PMCID: PMC12182525
- DOI: 10.1007/s10143-025-03673-0
Post-traumatic hydrocephalus after decompressive craniectomy: a multidimensional analysis of clinical, radiological, and surgical risk factors
Abstract
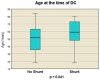
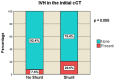
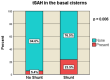
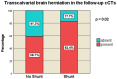
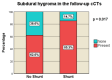
Decompressive craniectomy is a key treatment for refractory intracranial pressure after severe traumatic brain injury (TBI). Post-traumatic hydrocephalus (PTH) occurs in 7.6-36% of cases, and early diagnosis significantly improves rehabilitation outcomes. This retrospective study analyzed risk factors for shunt-dependent PTH in 126 TBI patients (93 men, 33 women, median age 53 years). Patients were divided into those requiring shunts and those who did not. Clinical and radiological characteristics, including volumetric measurements and surgical techniques, were assessed using SPSS® Statistics 25. The incidence of shunt-dependent PTH was 27%. Multivariate analysis identified significant risk factors: advanced age at craniectomy (p = 0.008; OR 1.048), traumatic subarachnoid hemorrhage in the basal cisterns (p = 0.015; OR 7.545), post-traumatic ischemic infarcts (p = 0.003; OR 5.319), transcalvarial brain herniation (p = 0.012; OR 5.543), subdural hygroma (p = 0.004; OR 8.131), and progression of contusion hemorrhages (p = 0.013; OR 4.386). Operative parameters did not show statistical significance. Neurological outcomes in shunt patients, assessed via the modified Rankin Scale and Extended Glasgow Outcome Scale, were significantly worse than in non-shunt patients (mRS > 3, GOS-E < 5, p = 0.001-0.011). Our findings suggest that subarachnoid hemorrhage in the cisterns, advanced age, hygromas, ischemic infarcts, transcalvarial herniation, and contusion hemorrhage progression are independent risk factors for shunt-dependent PTH. Additionally, shunt placement was linked to poorer neurological outcomes.
Not applicable.
Keywords: Decompressive craniectomy; Hydrocephalus; Post-traumatic hydrocephalus; Traumatic brain injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study, approved by the ethics committee of Technische Universität Dresden, was a retrospective observational analysis conducted at the University Hospital Dresden. Consent to participate: Not applicable. This study was a retrospective observational analysis. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Cisternostomy is not beneficial to reduce the occurrence of post-traumatic hydrocephalus in Traumatic Brain Injury.Acta Neurochir (Wien). 2024 Apr 30;166(1):200. doi: 10.1007/s00701-024-06084-0. Acta Neurochir (Wien). 2024. PMID: 38689141
-
Post-craniectomy hydrocephalus in adult traumatic brain injury patients: a systematic review and meta-analysis of risk factors and outcome.Neurosurg Rev. 2025 Jan 22;48(1):72. doi: 10.1007/s10143-025-03232-7. Neurosurg Rev. 2025. PMID: 39841279
-
Factors associated with shunt-dependent hydrocephalus after decompressive craniectomy for traumatic brain injury.J Neurosurg. 2018 May;128(5):1547-1552. doi: 10.3171/2017.1.JNS162721. Epub 2017 Jun 16. J Neurosurg. 2018. PMID: 28621627 Clinical Trial.
-
Development of Posttraumatic Hydrocephalus Requiring Ventriculoperitoneal Shunt After Decompressive Craniectomy for Traumatic Brain Injury: a Systematic Review and Meta-analysis of Retrospective Studies.Med Arch. 2018 Jun;72(3):214-219. doi: 10.5455/medarh.2018.72.214-219. Med Arch. 2018. PMID: 30061770 Free PMC article.
-
Hydrocephalus Renders Ventricular or Subarachnoid Drainage as an Efficacies Cure for Decompressive Craniectomy-Induced Subdural Effusion After Traumatic Brain Injury.J Craniofac Surg. 2025 Jul-Aug 01;36(5):1649-1654. doi: 10.1097/SCS.0000000000010819. Epub 2025 Mar 10. J Craniofac Surg. 2025. PMID: 40062940
References
-
- Anile C, De Bonis P, Di Chirico A, Ficola A, Mangiola A, Petrella G (2009) Cerebral blood flow autoregulation during intracranial hypertension: a simple, purely hydraulic mechanism? Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 25:325–335; discussion 337–340. 10.1007/s00381-008-0749-7 - PubMed
-
- Aschoff A, Kremer P, Hashemi B, Kunze S (1999) The scientific history of hydrocephalus and its treatment. Neurosurg Rev 22:67–93. 10.1007/s101430050035. discussion 94–95 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

