Ambient temperature transport of human oocytes: an unexpected research resource
- PMID: 40542920
- PMCID: PMC12423387
- DOI: 10.1007/s10815-025-03548-9
Ambient temperature transport of human oocytes: an unexpected research resource
Abstract
Purpose: This study aimed to determine the viability and meiotic competence of human oocytes deemed not suitable for clinical use following controlled ovarian stimulation of young egg donors receiving treatment at an egg bank.
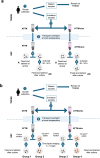
Methods: A total of 432 oocytes were shipped at ambient temperature overnight, in a medium containing caffeine and dibutyryl cyclic-AMP to limit meiotic cell cycle progression, and estrogen and progesterone to mimic the intrafollicular environment. In some experiments, transport medium was also supplemented with 1 µg/ml ZnSO4. Oocytes were either fixed immediately upon arrival or cultured for 20-24 or up to 143 h followed by fixation. Time-lapse imaging and fluorescence imaging were used to establish viability, meiotic status, and spontaneous activation.
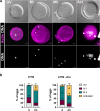
Results: Greater than 95% of transported oocytes retained viability, whether transported with or without added ZnSO4, exhibiting meiotic progression and/or spontaneous activation following overnight culture. Time-lapse imaging and fluorescence imaging revealed a higher incidence of spontaneous activation and subsequent cleavage activity for up to 5 days in culture in samples transported in ZnSO4.
Conclusions: Under the experimental conditions described here, immature human oocytes retain viability and meiotic competence following ambient temperature transport, providing a novel and experimentally tractable resource for future research in human oocyte biology and the development of human parthenote stem cells.
Keywords: Ambient temperature transport; Human oocyte; Parthenote stem cells; Spontaneous activation; Zinc.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: All TWESB oocyte donors signed an oocyte donation request form that included a stipulation that oocytes deemed unsuitable for cryopreservation could be used for this research. The consent form was also reviewed and approved by the Ethics Advisory Board and Human Subjects Committee of the Bedford Research Foundation. Conflict of interest: The authors declare no competing interests.
Figures


References
-
- Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Ernst E, Macklon KT, et al. Fertility preservation for age-related fertility decline. Lancet. 2015;385(9967):506–7. - PubMed
-
- Martin JR, Bromer JG, Sakkas D, Patrizio P. Live babies born per oocyte retrieved in a subpopulation of oocyte donors with repetitive reproductive success. Fertil Steril. 2010;94(6):2064–8. - PubMed
-
- Sabbagh R, Mulligan S, Shah J, Korkidakis A, Penzias A, Vaughan D, et al. From oocytes to a live birth: are we improving the biological efficiency? Fertil Steril. 2023;120(6):1210–9. - PubMed
-
- Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998;4(2):103–20. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

