Novel Potassium Binders in Reduction of Hyperkalemia and Optimization of RAAS Inhibitors Treatment in Patients with Chronic Kidney Disease or Heart Failure: A Systematic Review and Meta-analysis
- PMID: 40542996
- PMCID: PMC12321686
- DOI: 10.1007/s40265-025-02198-6
Novel Potassium Binders in Reduction of Hyperkalemia and Optimization of RAAS Inhibitors Treatment in Patients with Chronic Kidney Disease or Heart Failure: A Systematic Review and Meta-analysis
Abstract
Background: Renin-angiotensin-aldosterone system inhibitors (RAASi) and its combination therapy are essential supportive treatments for patients with chronic kidney disease (CKD) and heart failure (HF). Hyperkalemia (HK) is the major safety concern associated with the treatments, which often leads to RAASi dose reduction or discontinuation, thereby compromising cardiovascular protective effects. Although novel potassium binders (NPBs) are recommended by current guidelines for the treatment of HK, systematic evidence is needed to guide their use in RAASi optimization and HK management. A systematic review and meta-analysis was conducted to evaluate the incidence of HK in patients with CKD or HF who received RAASi and the efficacy of NPBs in RAASi optimization.
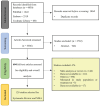
Methods: PubMed, Medline, Embase, and the Cochrane Library were searched from January 1, 2011 to December 31, 2023. Any studies of adult patients with CKD or HF who received RAASi were included in systematic review of HK incidence and risk factors (part 1). Randomized controlled trials (RCTs) of NPBs in patients with CKD or HF were included in meta-analysis on the efficacy of novel potassium binders (NPBs) (part 2). The primary outcome was optimization of RAASi therapy with NPBs. A pooled analysis was conducted in part 1. Network meta-analyses using a random-effects model were performed in part 2.
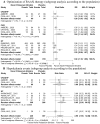
Results: A total of 83 studies (24 with CKD, 54 with HF, and 5 with CKD and HF) were included in part 1 and 8 RCTs (2 with CKD, 4 with HF, and 2 with CKD and HF) were included in part 2. The pooled HK incidence was 10.7% overall at any criteria and in all patients who received RAASi. The highest incidence of HK was observed with the combination of angiotensin converting enzyme inhibitor (ACEi)/angiotensin ii receptor blockers (ARBs)/angiotensin receptor-neprilysin inhibitor (ARNi)+ mineralocorticoid receptor antagonists (MRAs) (24.4%), followed by the triple therapy ACEi/ARB/ARNi+ sodium glucose cotransporter-2 inhibitor (SGLT2i)+MRA (10.9%), and ACEi/ARB/ARNi + SGLT2i (2.2%). Novel potassium binders improved RAASi optimization by 38% compared with placebo (risk ratio, 1.38; 95% CI, 1.16-1.65). Additionally, NPBs decreased the incidence of HK by 28% and reduced the level of potassium by 0.71 mEq/L. The CKD population showed a higher optimization rate than the HF population (84% vs 29%).
Conclusion: The RAASi treatment was associated with high prevalence of HK, especially in bigeminal and triple therapy. The NPBs were effective in RAASi optimization and HK management, especially among the CKD population.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This work was supported by grants from the National Natural Science Foundation of China (Nos. 82170737, 82200820, and 82370707), NHC Key Laboratory of Clinical Nephrology (Sun Yat-Sen University), Guangzhou Science and Technology Project (No. 2025A04J5292, 202206080010), Guangdong Provincial Key Laboratory of Nephrology, Guangdong International Science and Technology Cooperation Institute of Immune Kidney Disease and Precision Medicine, Guangdong Basic and Applied Basic Research Foundation (No. 2023B0303000013), and 2024 Guangzhou Science and Technology Fund for Agriculture and Social Development Special Topic (No. 2024B03J1337). This work also received funding from AstraZeneca. Conflict of Interest: The authors (NH, YX, CL, YL, YF, ZL, DZ, HM, WC) declare no conflicts of interest. Ethics Approval: Not applicable. Consent to Participate: Not applicable. Code Availability: Not applicable. Consent for Publication: Not applicable. Availability of Data and Materials: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Author Contributions: NH, YX, CL, DZ, HM, and WC conceived and designed the study. NH, YX, CL, YL, YF, ZL, and DZ screened and selected the articles. NH, YX, CL, YF, and ZL extracted the data. NH, YX, CL, YF, and ZL assessed the risk of bias, NH, CL, YF, and ZL analyzed the data, HM, and WC supervised the data analyses, NH, and CL rated the certainty of evidence, NH, YX, and WC interpreted the data, NH, CL, YF, and ZL drafted the manuscript. All authors had full access to all the data in the study, had reviewed and approved the final version of the manuscript, and agree to be accountable for the work.
Figures






Similar articles
-
Renin-Angiotensin-Aldosterone System Inhibitor Dosing After Initiation of Outpatient Sodium Zirconium Cyclosilicate Therapy: The GALVANIZE RAASi Real-World Evidence Study.Adv Ther. 2025 Aug;42(8):3960-3977. doi: 10.1007/s12325-025-03254-z. Epub 2025 Jun 18. Adv Ther. 2025. PMID: 40531443 Free PMC article.
-
Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction.Cochrane Database Syst Rev. 2018 Jun 28;6(6):CD012721. doi: 10.1002/14651858.CD012721.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2021 May 22;5:CD012721. doi: 10.1002/14651858.CD012721.pub3. PMID: 29952095 Free PMC article. Updated.
-
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease.Cochrane Database Syst Rev. 2011 Oct 5;(10):CD007751. doi: 10.1002/14651858.CD007751.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2023 Jul 19;7:CD007751. doi: 10.1002/14651858.CD007751.pub3. PMID: 21975774 Updated.
-
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease.Cochrane Database Syst Rev. 2023 Jul 19;7(7):CD007751. doi: 10.1002/14651858.CD007751.pub3. Cochrane Database Syst Rev. 2023. PMID: 37466151 Free PMC article.
-
Effects of sodium-glucose cotransporter-2 inhibitors and aldosterone antagonists, in addition to renin-angiotensin system antagonists, on major adverse kidney outcomes in patients with type 2 diabetes and chronic kidney disease: A systematic review and network meta-analysis.Diabetes Obes Metab. 2022 Nov;24(11):2159-2168. doi: 10.1111/dom.14801. Epub 2022 Jul 15. Diabetes Obes Metab. 2022. PMID: 35712807
References
-
- Hundemer GL, Sood MM. Hyperkalemia with RAAS inhibition: mechanism, clinical significance, and management. Pharmacol Res. 2021;172: 105835. 10.1016/j.phrs.2021.105835. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. 10.1016/j.jacc.2017.04.025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Nos. 82170737/National Natural Science Foundation of China
- 82370707/National Natural Science Foundation of China
- 82200820/National Natural Science Foundation of China
- No. 2025A04J5292/Guangzhou Science and Technology Project
- 202206080010/Guangzhou Science and Technology Project
- No. 2023B0303000013/Guangdong Provincial Key Laboratory of Nephrology, Guangdong International Science and Technology Cooperation Institute of Immune Kidney Disease and Precision Medicine, Guangdong Basic and Applied Basic Research Foundation
- No. 2024B03J1337/2024 Guangzhou Science and Technology Fund for Agriculture and Social Development Special Topic
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

