Mechanistic Insights into Lipooligourea-Lipid Membrane Interactions
- PMID: 40543090
- PMCID: PMC12235613
- DOI: 10.1021/acs.jpcb.5c02112
Mechanistic Insights into Lipooligourea-Lipid Membrane Interactions
Abstract
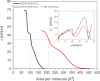
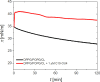
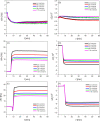
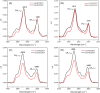
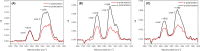
Understanding how synthetic peptidomimetics interact with bacterial membranes is key to developing next-generation antimicrobials. In this study, we investigate the membrane-disruptive behavior of C10-OU4, a cationic lipooligourea foldamer that mimics the amphiphilic architecture of antimicrobial lipopeptides. Using a multitechnique approach─Langmuir monolayer analysis, quartz crystal microbalance with dissipation monitoring (QCM-D), and attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR)─we probe the concentration-dependent interactions of C10-OU4 with lipid membranes that model Gram-positive bacterial membranes. At low concentrations (1 μM), C10-OU4 adsorbs to the membrane surface, inducing minor structural perturbations limited to the polar headgroup region. Increasing the concentration to 5 μM results in significant acyl chain disorder, partial membrane solubilization, and likely, micelle-like aggregate formation, as evidenced by QCM-D frequency shifts and ATR-FTIR data. At 10 μM, near the minimal inhibitory concentration, membrane disintegration becomes extensive, with the lipooligourea adopting orientations suggestive of random or tilted insertion geometries. These findings support a multimodal mechanism of action that transitions from surface association to full bilayer disruption in a concentration-dependent manner. The combined use of structural and dynamic measurements provides detailed insight into the physicochemical principles underlying lipooligourea-membrane interactions, offering a foundation for the rational design of membrane-active foldamer antibiotics.
Figures
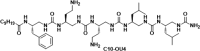
Similar articles
-
Headgroup-driven binding selectivity of alkylphospholipids to anionic lipid bilayers.Colloids Surf B Biointerfaces. 2025 Jul 17;255:114964. doi: 10.1016/j.colsurfb.2025.114964. Online ahead of print. Colloids Surf B Biointerfaces. 2025. PMID: 40695077
-
Cationic Proteins Rich in Lysine Residue Trigger Formation of Non-bilayer Lipid Phases in Model and Biological Membranes: Biophysical Methods of Study.J Membr Biol. 2023 Dec;256(4-6):373-391. doi: 10.1007/s00232-023-00292-y. Epub 2023 Sep 21. J Membr Biol. 2023. PMID: 37735238 Review.
-
Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease.Cochrane Database Syst Rev. 2001;(3):CD003234. doi: 10.1002/14651858.CD003234. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003234. doi: 10.1002/14651858.CD003234.pub2. PMID: 11687058 Updated.
-
Prophylactic antibiotics for preventing gram-positive infections associated with long-term central venous catheters in adults and children receiving treatment for cancer.Cochrane Database Syst Rev. 2021 Oct 7;10(10):CD003295. doi: 10.1002/14651858.CD003295.pub4. Cochrane Database Syst Rev. 2021. PMID: 34617602 Free PMC article.
-
Adjunctive steroid therapy versus antibiotics alone for acute endophthalmitis after intraocular procedure.Cochrane Database Syst Rev. 2017 Feb 22;2(2):CD012131. doi: 10.1002/14651858.CD012131.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Jun 6;6:CD012131. doi: 10.1002/14651858.CD012131.pub3. PMID: 28225198 Free PMC article. Updated.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

