CryoEM structure of Rv2531c reveals cofactor-induced tetramer-dimer transition in a tuberculin amino acid decarboxylase
- PMID: 40543586
- PMCID: PMC12329521
- DOI: 10.1016/j.jbc.2025.110394
CryoEM structure of Rv2531c reveals cofactor-induced tetramer-dimer transition in a tuberculin amino acid decarboxylase
Abstract
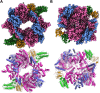
The survival of Mycobacteriumtuberculosis relies on its ability to adapt to dynamic and hostile host environments. Amino acid decarboxylases play a crucial role in these adaptations, but their structural and mechanistic properties are not fully understood. Bioinformatic analyses revealed that these enzymes exist in three distinct forms based on their domain organization. We used cryoEM at 2.76 Å resolution to show that Rv2531c exhibits unexpected oligomeric and conformational flexibility. The enzyme forms a tetramer with distinct open and closed conformations in its apo state, suggesting dynamic intersubunit interactions. Upon binding pyridoxal 5'-phosphate, the enzyme undergoes a dramatic structural rearrangement, transitioning into a dimer. These findings reveal a novel mechanism of oligomeric plasticity. We also uncover an amino-terminal domain that might play a role in this process. Our results provide critical insights into the structural adaptations that support bacterial persistence under intracellular stress. By elucidating the apo and pyridoxal 5'-phosphate-bound states of Rv2531c, we contribute to a deeper understanding of how M. tuberculosis navigates its challenging intracellular environment. These insights into the unique structural features of Rv2531c offer a foundation for targeting metabolic resilience in tuberculosis and open avenues for future studies on the role of this domain in pathogenesis.
Keywords: Mycobacterium tuberculosis; cryogenic electron microscopy; glutamate decarboxylase; pyridoxal 5'-phosphate; γ-aminobutyric acid.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Stigma Management Strategies of Autistic Social Media Users.Autism Adulthood. 2025 May 28;7(3):273-282. doi: 10.1089/aut.2023.0095. eCollection 2025 Jun. Autism Adulthood. 2025. PMID: 40539215
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
Factors that influence caregivers' and adolescents' views and practices regarding human papillomavirus (HPV) vaccination for adolescents: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2025 Apr 15;4(4):CD013430. doi: 10.1002/14651858.CD013430.pub2. Cochrane Database Syst Rev. 2025. PMID: 40232221 Free PMC article.
References
-
- Seebeck F.P. In vitro reconstitution of Mycobacterial ergothioneine biosynthesis. J. Am. Chem. Soc. 2010;132:6632–6633. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

