TSC22 domain family member 3 links natural killer cells to CD8+ T cell-mediated drug hypersensitivity
- PMID: 40544157
- PMCID: PMC12182574
- DOI: 10.1038/s41392-025-02300-0
TSC22 domain family member 3 links natural killer cells to CD8+ T cell-mediated drug hypersensitivity
Abstract
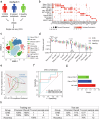
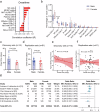
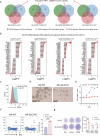
Severe cutaneous adverse drug reactions (SCARs) are life-threatening diseases, which are associated with human leukocyte antigen (HLA) risk variants. However, the low positive predictive values of HLA variants suggest additional factors influence disease susceptibility. Using dapsone hypersensitivity syndrome (DHS) as a paradigm for SCARs, we show that the DHS patients harbor a sex-related global reduction in blood NK cells, contributing to the higher incidence of reactions in females. Single-cell RNA sequencing revealed a decrease in the immunoregulatory CD56low XCL1/2low NK cell subset and an expansion of CD56high XCL1/2high NK cell subsets with an effector phenotype in DHS patients compared to dapsone-tolerant individuals. Functionally, interleukin-15 superagonist-induced activation of NK cells exacerbated SCARs-like symptoms in a murine model. Mechanistically, TSC22 domain family member 3 (TSC22D3) deficiency enhanced NK cell effector function, shifting the immune response from CD4+ T cell to CD8+ T cell function. These results demonstrate that TSC22D3-regulated NK cells play a critical role in predisposing to drug hypersensitivity reactions, bridging innate and adaptive immune dysregulation in SCARs pathogenesis. Our study highlights the importance of NK cell heterogeneity and TSC22D3 in immune-mediated hypersensitivity disorders, offering potential therapeutic targets for SCARs and related conditions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Kroshinsky, D., Cardones, A. R. G. & Blumenthal, K. G. Drug Reaction with Eosinophilia and Systemic Symptoms. N. Engl. J. Med. 391, 2242–2254 (2024). - PubMed
-
- Saag, M. et al. High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin. Infect. Dis.46, 1111–1118 (2008). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

