Truncated LKB1 nonenzymatically enhances Fas-induced apoptosis by acting as a surrogate of Smac
- PMID: 40544190
- PMCID: PMC12182575
- DOI: 10.1038/s41420-025-02570-1
Truncated LKB1 nonenzymatically enhances Fas-induced apoptosis by acting as a surrogate of Smac
Abstract
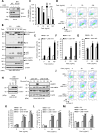
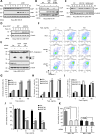
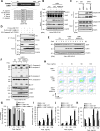
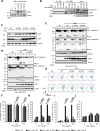
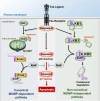
Although liver kinase B1 (LKB1) has been established as a tumor suppressor kinase, its mechanism of action is incompletely understood. Here we describe a novel nonenzymatic function of LKB1 in cell death induced by Fas/CD95. In BID knockout HeLa cells, inactivation of mitochondrial outer membrane permeabilization (MOMP) prevents Smac-induced inhibition of X-linked inhibitor of apoptosis (XIAP), causing resistance to Fas-induced apoptosis. However, reexpression of LKB1 in those cells naturally deficient for endogenous LKB1 restored apoptosis. Mechanistically, caspase-8 activated by Fas processed LKB1 to a truncated form, tLKB1. Both WT and kinase-inactive LKB1 antagonized XIAP to restore apoptosis, but somatic mutants of LKB1 found in Peutz-Jeghers syndrome (PJS) failed to do so. Thus, in addition to the known caspase-8 / tBid / Smac / XIAP pro-apoptotic axis, our results unveil a novel one, caspase-8 / tLKB1 / XIAP that potentially contributes to the antitumor functions of LKB1.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval and consent to participate: All methods were performed in accordance with the relevant guidelines and regulations. Recombinant DNA experiments were approved by ethics committee of Tohoku University (approval number: 2018PhR-019-02). All authors checked the study and agreed to participate in the manuscript. Consent for publication: All authors checked the study and agreed.
Figures

References
-
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. - PubMed
-
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Bio. 2008;9:231–41. - PubMed
-
- Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. - PubMed
-
- Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet. 1999;33:29–55. - PubMed
Grants and funding
- JP24K02237/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP21H02691/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP24KJ0428/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP18J20440/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP24K02173/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

