Chiral phosphoric acid-catalyzed atroposelective iodination of N-arylindoles
- PMID: 40544218
- PMCID: PMC12182571
- DOI: 10.1038/s42004-025-01584-1
Chiral phosphoric acid-catalyzed atroposelective iodination of N-arylindoles
Abstract
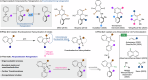
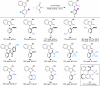
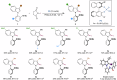
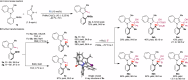
Axially chiral indoles have garnered significant attention due to their synthetic and biological importance. However, atroposelective halogenation of this scaffold has been rarely explored. This study presents a catalytic and enantioselective iodination of N-arylindoles, achieving precise control over the C-N stereogenic axis. The reaction is facilitated by a chiral phosphoric acid, which promotes iodination at the C-3 position of N-arylindole, followed by iodine migration and deprotonation. A hydrogen-bonding donor on the aromatic ring plays a key role in achieving high enantioselectivities. Under optimized reaction conditions, a wide range of substrates are well-tolerated, and subsequent reactions with various carbonyl electrophiles maintain enantioselectivity. Computational studies provide insights into the origin of enantioselectivity at the plausible enantiodetermining step.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Differently different?: A commentary on the emerging social cognitive neuroscience of female autism.Biol Sex Differ. 2024 Jun 13;15(1):49. doi: 10.1186/s13293-024-00621-3. Biol Sex Differ. 2024. PMID: 38872228 Free PMC article. Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
-
- Liu, Y., Leng, H.-J., Li, Q.-Z. & Li, J.-L. Catalytic strategies for the asymmetric construction of cyclic frameworks with a halogenated tetrasubstituted stereocenter. Adv. Synth. Catal.362, 3926–3947 (2020).
-
- Wong, J. & Yeung, Y.-Y. Recent advances in C–Br bond formation. Synlett32, 1354–1364 (2021).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

