An imaging-guided self-amplifying photo-immunotherapeutic nanoparticle for STING pathway activation and enhanced cancer therapy
- PMID: 40544257
- PMCID: PMC12181854
- DOI: 10.1186/s12951-025-03536-2
An imaging-guided self-amplifying photo-immunotherapeutic nanoparticle for STING pathway activation and enhanced cancer therapy
Abstract
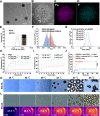
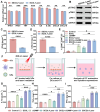
The stimulator of interferon genes (STING) pathway is a promising target in cancer immunotherapy. However, current nanomedicine strategies targeting the STING pathway often suffer from limited tumor specificity and insufficient immune activation. In this study, we developed a novel imaging-guided, self-amplifying photo-immunotherapeutic nanoparticle (SSCOL), comprising a liposome framework that encapsulates the phase-change material perfluoropentane (PFP), the photothermal agent superparamagnetic iron oxide (SPIO), and the STING agonist cGAMP. This nanoparticle exhibits excellent photoacoustic/ultrasound dual-modal imaging capability, enabling precise visualization of tumor tissue. CREKA enables specific binding to fibrin-fibronectin complexes in the tumor stroma, while NIR-induced photothermal effects of SPIO trigger coagulation, amplifying target formation and enhancing nanoparticle accumulation via a positive feedback mechanism. Under photothermal therapy, the phase transition of SSCOL enables the controlled and efficient release of the encapsulated cGAMP, which subsequently activates the STING pathway and triggers a pro-inflammatory cascade, enhances dendritic cell maturation and cytotoxic T lymphocyte activation, and elicits robust immune responses against both primary and metastatic tumors. Collectively, this multifunctional nanoparticle offers a promising strategy that integrates imaging, targeting, and photothermal-enhanced immune activation for STING-mediated cancer immunotherapy.
Keywords: Dual-modal imaging; Immunotherapy; Photothermal therapy; STING pathway; Targeting.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal experiments in this study were approved by the Animal Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University (Coren Trial(265), 2021) and conducted at the Laboratory Animal Center of Chongqing Medical University. Consent for publication: All the authors have approved the manuscript to publish. Competing interests: The authors declare no competing interests.
Figures








Similar articles
-
LASIP: Light-Activated STING Immunotherapeutic Patch: A Multifunctional Microneedle Platform for Combinatorial Mild Hyperthermia with Immunotherapy in Breast Cancer.ACS Appl Mater Interfaces. 2025 Jul 2;17(26):37806-37825. doi: 10.1021/acsami.5c10509. Epub 2025 Jun 17. ACS Appl Mater Interfaces. 2025. PMID: 40527606
-
iRGD-NaGd(MoO4)2: A Tumor-Targeted Magnetic Resonance Contrast Agent Activating Immunotherapy via the cGAS-STING Pathway.ACS Appl Mater Interfaces. 2025 Jul 2;17(26):37448-37464. doi: 10.1021/acsami.5c03469. Epub 2025 Jun 19. ACS Appl Mater Interfaces. 2025. PMID: 40538146
-
Dual-modality immune nano-activator harnessing Mn2⁺ and quercetin to potentiate the cGAS-STING pathway for advanced cancer metalloimmunotherapy.J Nanobiotechnology. 2025 Mar 25;23(1):248. doi: 10.1186/s12951-025-03336-8. J Nanobiotechnology. 2025. PMID: 40128784 Free PMC article.
-
The cGAS-STING pathway in cancer immunity: dual roles, therapeutic strategies, and clinical challenges.Essays Biochem. 2025 Mar 7;69(2):EBC20253006. doi: 10.1042/EBC20253006. Essays Biochem. 2025. PMID: 40052963 Free PMC article. Review.
-
Activation of the cGAS-sting Pathway Mediated by Nanocomplexes for Tumor Therapy.Curr Pharm Des. 2025;31(20):1604-1618. doi: 10.2174/0113816128339788241221160639. Curr Pharm Des. 2025. PMID: 39819537 Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. - PubMed
-
- Yalamandala BN, Moorthy T, Liu ZH, Huynh TMH, Iao HM, Pan WC, Wang KL, Chiang CS, Chiang WH, Liao LD, et al. A Self-Cascading catalytic therapy and antigen capture Scaffold-Mediated T cells augments for postoperative brain immunotherapy. Small. 2025;21:e2406178. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

