Inhibition of the ATP synthase c subunit ameliorates HDM/LPS-induced inflammatory responses in asthmatic bronchial epithelial cells by blocking the mPTP-mtDNA-cGAS-STING axis
- PMID: 40544259
- PMCID: PMC12181862
- DOI: 10.1186/s12931-025-03299-2
Inhibition of the ATP synthase c subunit ameliorates HDM/LPS-induced inflammatory responses in asthmatic bronchial epithelial cells by blocking the mPTP-mtDNA-cGAS-STING axis
Abstract
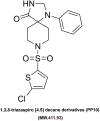
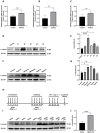
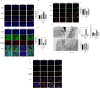
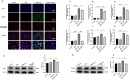
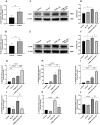
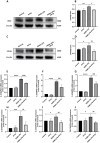
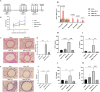
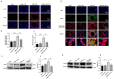
The ATP synthase c subunit (c subunit) constitutes the mitochondrial permeability transition pore (mPTP). The extended opening of the mPTP is crucial in the development of various human illnesses. Nevertheless, it remains unclear whether the c subunit regulates the prolonged opening of the mPTP to attenuate inflammatory responses in asthma. This study sought to clarify the impact of the c subunit on inflammatory responses and to examine the therapeutic effects of 1,3,8-triazaspiro [4.5] decane derivatives (PP10), a c subunit inhibitor, in human bronchial epithelial (HBE) cells induced by house dust mite (HDM) and lipopolysaccharide (LPS), as well as in a mouse model. The findings indicated that the expression of the c subunit is elevated in asthmatic patients, HDM/LPS-induced HBE cells, and asthmatic mice. The inhibition of the c subunit by PP10 alleviated the prolonged opening of mPTP, then blocked the release of mitochondrial DNA (mtDNA) and cyclic GMP-AMP synthase (cGAS)-interferon response cGAMP interactor (STING) pathway activation in HDM/LPS-induced HBE cells. Furthermore, PP10 decreased the secretion of inflammatory cytokines and ameliorated airway inflammation in HDM/LPS-induced HBE cells and asthmatic animals, respectively. The data collectively suggest that the c subunit triggers an inflammatory response by promoting the sustained opening of mPTP, leading to the activation of the mtDNA-GAS-STING pathway in HDM/LPS-induced HBE cells. Inhibition of the c-subunit attenuates inflammatory responses in HDM/LPS-induced cells or mouse models. Clinical trial number Not applicable.
Keywords: ATP synthase c subunit; Airway epithelial cells; Asthma; Inflammatory responses; Mitochondria; Mitochondrial permeability transition pore.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The deployment of human medical records and biological samples from human subjects was conducted in accordance with the tenets set forth in the Declaration of Helsinki. Informed consent was obtained from all participants. All experimental protocols related to human samples and animal experiments were approved by the Ethics Committee of Tongji Hospital affiliated with Tongji Medical College of Huazhong University of Science and Technology (TJ-IRB202404061 and TJH-202310017). Consent to publish: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

