Weight loss with real-world doravirine use in the OPERA cohort: a US-based cohort study
- PMID: 40544263
- PMCID: PMC12182698
- DOI: 10.1186/s12981-025-00761-5
Weight loss with real-world doravirine use in the OPERA cohort: a US-based cohort study
Abstract
Background: Weight gain has been associated with the use of antiretrovirals in people with HIV, especially with integrase inhibitors or tenofovir alafenamide, and among women. In 2018, doravirine became the latest non-nucleoside reverse transcriptase inhibitor to be approved in the US. We assessed changes in weight over time among virologically suppressed individuals who switched to a regimen containing doravirine (DOR).
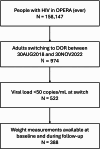
Methods: From the US-based OPERA cohort, treatment-experienced adults with HIV who switched to a DOR-containing regimen between 30AUG2018-30NOV2022 with a viral load < 50 copies/mL were included (followed through 31MAY2023). The study population was characterized and a linear mixed model was used to estimate rates of weight change on DOR. Results were stratified by sex, by patterns of efavirenz (EFV) and/or tenofovir disoproxil fumarate (TDF) use before/after switch to DOR, and by integrase inhibitor (INSTI) & tenofovir alafenamide (TAF) use combination (restricted to individuals who maintained the same combination before/after switch).
Results: Of 388 included individuals, 21% were women, 33% were Black, and 78% were obese or overweight at DOR switch. Overall, people who switched to DOR lost an average of 0.80 kg/year (95% CI: -1.32, -0.28). Both women and men experienced statistically significant weight loss; women (70% Black, 70% aged ≥ 40 years) lost weight at a rate of -1.67 kg/year (95% CI: -3.32, -0.02) and men at a rate of -0.60 kg/year (95% CI: -1.12, -0.08). When EFV and TDF were absent before and after switch to DOR, statistically significant weight loss was observed. Among those who had the same INSTI and TAF combination throughout and had any INSTI or TAF use, a statistically non-significant trend toward weight loss was observed.
Conclusions: In one of the first real-world analyses of weight changes among virologically suppressed individuals who switched to a DOR-containing regimen in the US, DOR was associated with statistically significant weight loss. Patterns of use of other antiretrovirals did not fully explain the observed weight loss. These findings are clinically meaningful given that most individuals included were overweight or obese at switch to DOR and that women were predominantly of perimenopausal or menopausal age.
Keywords: Cohort; Doravirine; Durability; HIV; Real-world evidence; Weight.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Institutional review board (IRB) approval covering patient data contained in the OPERA database was received from Advarra IRB; a waiver of informed consent and authorization for the use of protected health information for patient data was granted (Pro00023648). The study was conducted in accordance with HIPAA and HITECH requirements, which expand upon the ethical principles detailed in the 1964 Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: KM has received research grants from Gilead Sciences, Merck, Janssen, and GSK/ViiV Healthcare and honoraria for Speakers Bureau and Advisory Boards from Gilead Sciences, Merck, Janssen and GSK/ViiV Healthcare; and advisory board participation with Epividian. LB, MDO, JSF, and GPF are employed by Epividian, Inc.; Epividian has had research funded by AIDS Healthcare Foundation, EMD Serono, Gilead Sciences, Janssen Scientific Affairs, LLC, Merck & Co., Theratechnologies Inc., and ViiV Healthcare. MS is on the Speakers Bureau for ViiV Healthcare and Gilead Sciences, and on the advisory board for ViiV Healthcare and Epividian. RKH has received research grants from Gilead Sciences, Janssen, and ViiV Healthcare, speaker honoraria from ViiV Healthcare, Merck, Gilead Sciences, and Janssen, and advisory board participation with ViiV Healthcare, Gilead Sciences, Janssen, and Epividian. YOW is employed by Merck & Co., Inc. Abbreviations: µl Microliter. 3TC Lamivudine. AIDS Acquired immunodeficiency syndrome. ARV Antiretroviral. BMI Body mass index. CI Confidence interval. dL Deciliter. DOR Doravirine. EFV Efavirenz. eGFR Estimated glomerular filtration rate. FDA Food and Drug Administration. HIPAA Health Insurance Portability and Accountability Act. HITECH Health Information Technology for Economic and Clinical Health. HIV Human immunodeficiency virus. INSTI Integrase strand transfer inhibitor. IQR Interquartile range. IRB Institutional Review Board. kg Kilogram. N Number. NNRTI Non-nucleoside reverse transcriptase inhibitor. NRTI Nucleoside reverse transcriptase inhibitor. OPERA Observational Pharmaco-Epidemiology Research and Analysis. PI Protease inhibitor. TAF Tenofovir alafenamide. TDF Tenofovir disoproxil fumarate. US United States.
Figures

References
-
- UNAIDS. UNAIDS Data. 2021. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2021. Contract No.: Licence: CC BY-NC-SA 3.0 IGO.
-
- Surial B, Mugglin C, Calmy A, Cavassini M, Gunthard HF, Stockle M et al. Weight and metabolic changes after switching from Tenofovir disoproxil fumarate to Tenofovir Alafenamide in people living with HIV: A cohort study. Ann Intern Med. 2021. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

