3D-printed magnesium/strontium-co-doped calcium silicate scaffolds promote angiogenesis and bone regeneration through synergistic bioactive ion stimulation
- PMID: 40544274
- PMCID: PMC12182697
- DOI: 10.1186/s13036-025-00528-6
3D-printed magnesium/strontium-co-doped calcium silicate scaffolds promote angiogenesis and bone regeneration through synergistic bioactive ion stimulation
Abstract
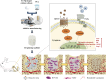
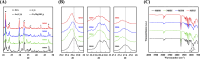
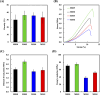
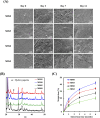
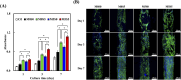
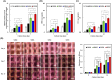
Bone defects resulting from trauma, infection, or surgical resection require biomaterials that support osteogenesis and vascularization for effective regeneration. In this study, we developed a 3D-printed magnesium- and strontium-co-doped calcium silicate (MSCS) scaffold using direct ink writing to optimize its bioactivity and structural integrity. X-ray diffraction confirmed the successful incorporation of Sr and Mg, leading to phase modifications that influenced ion release and degradation. Wettability and mechanical testing showed that Sr improved the stability, while Mg accelerated degradation, with M5S5 co-doping exhibiting a balanced degradation profile. In vitro, Wharton's jelly mesenchymal stromal cells cultured on M5S5 scaffolds displayed enhanced proliferation, cytoskeletal organization, and osteogenic differentiation, as evidenced by increased alkaline phosphatase activity and bone matrix protein expression. Angiogenesis assays using human umbilical vein endothelial cells revealed that Sr and Mg co-doping synergistically enhanced vascular endothelial growth factor and angiopoietin-1 secretion, thereby promoting endothelial tube formation. In vivo micro-computed tomography and histological analysis of a rabbit femoral defect model confirmed that M5S5 facilitated extensive new bone formation, exhibiting superior trabecular architecture and mineralization. These findings highlight MSCS scaffolds as promising biomaterials for bone tissue engineering applications.
Keywords: 3D printing; Bone regeneration; Calcium silicate; Magnesium; Strontium.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures






References
-
- Liu Z, He X, Chen S, Yu H. Advances in the use of calcium silicate-based materials in bone tissue engineering. Ceram Int. 2023;49:19355–63. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

