DC-SIGN (CD209)-mediated interactions between bacteria, lung cancer tissues, and macrophages promote cancer metastasis
- PMID: 40544275
- PMCID: PMC12181914
- DOI: 10.1186/s13027-025-00667-x
DC-SIGN (CD209)-mediated interactions between bacteria, lung cancer tissues, and macrophages promote cancer metastasis
Abstract
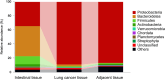
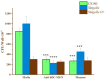
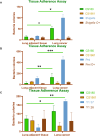
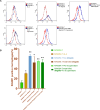
One of the hallmarks of lung cancers is the earlier metastasis resulting from the dissemination of cancer cells. Although accumulating evidence suggests that bacterial infection may be involved in the development of the metastasis of lung cancer, few studies have explored the molecular mechanisms of bacterial infection in the dissemination of lung cancer cells. A series of studies have indicated that certain Gram-negative bacteria are able to hijack antigen-presenting cells (APCs) via interaction with DC-SIGN (CD209) receptors to facilitate the dissemination of pathogens, including viruses, bacteria, fungi, and parasites. Therefore, in the present work, it was hypothesized that bacterial infection may promote the dissemination of cancer cells via the utilization of a similar mechanism. It was first discovered that human lung cancer tissues contain a very high diversity of bacterial DNAs, indicating the co-existence of lung cancer tissues and microbial organisms. It was then found that lung cancer tissues express DC-SIGN, leading to binding with a Gram-negative bacterium, Shigella sonnei. Further, this bacterium was found to be able not only to induce the expression of DC-SIGN on macrophages but also to enhance the migration ability of lung cancer cells in vitro. The in vivo experiments supported these observations, showing that in wild-type (WT) mice, Shigella sonnei infection significantly increased tumor size, weight, and metastatic nodules compared to SIGNR1 knockout (KO) mice. These observations were associated with increasing DC-SIGN expression in WT mice. Finally, these results suggest that bacterial infections could play a significant role in promoting lung cancer progression and metastasis via DC-SIGN-mediated mechanisms.
Keywords: DC-SIGN; Gram-negative bacteria; Lung cancer; Metastasis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2018 Feb 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. doi: 10.1002/14651858.CD008649.pub4. PMID: 29424930 Free PMC article. Updated.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher's disease: a systematic review.Health Technol Assess. 2006 Jul;10(24):iii-iv, ix-136. doi: 10.3310/hta10240. Health Technol Assess. 2006. PMID: 16796930
References
-
- Schwartz, A.G. and M.L. Cote, Epidemiology of lung cancer. Lung cancer and personalized medicine: Current knowledge and therapies, 2016: p. 21–41.
-
- Oberndorfer F, Müllauer L. Molecular pathology of lung cancer: current status and perspectives. Curr Opin Oncol. 2018;30(2):69–76. - PubMed
-
- NaSiM F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am. 2019;103:463–73. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

