Integrated peripheral blood multi-omics profiling identifies immune signatures predictive of neoadjuvant PD-1 blockade efficacy in head and neck squamous cell carcinoma
- PMID: 40544277
- PMCID: PMC12182656
- DOI: 10.1186/s12967-025-06770-2
Integrated peripheral blood multi-omics profiling identifies immune signatures predictive of neoadjuvant PD-1 blockade efficacy in head and neck squamous cell carcinoma
Abstract
Background: Neoadjuvant PD-1 inhibitor therapy has shown promise in locally advanced head and neck squamous cell carcinoma (HNSCC), but only a subset of patients achieves major pathological responses. Liquid biopsy, the analysis of tumor-derived biomarkers in readily accessible bodily fluids (primarily blood), offers significant advantages over traditional tissue biopsies for predicting cancer treatment outcomes. The aim of this study is to develop a predictive model for neoadjuvant PD-1 therapy response in HNSCC patients using exclusively liquid biopsy approaches-namely, peripheral blood immune profiling (CyTOF) and plasma cytokine panels (Olink).
Methods: In a prospective trial involving 50 HNSCC patients treated with neoadjuvant tislelizumab plus chemotherapy, peripheral blood samples were collected pre- and post-treatment. Immune cell subsets were analyzed by mass cytometry (CyTOF), and circulating protein markers were quantified via a 92-plex targeted proteomics panel (Olink). Multimodal features were integrated into a predictive model using logistic regression.
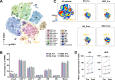
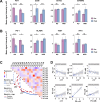
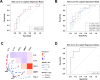
Results: Baseline immune profiles differed significantly between responder (RD) and non-responder (NRD): RD showed higher frequencies of CD103-CD8+ central memory T cells (Tcm, c03) and elevated plasma interleukins (IL-5, IL-13), whereas NRD had more CD28-TIGIThighcPARP-CD8+ terminally differentiated effector memory CD45RA+ T cells (Temra, c17) and higher levels of chemokines (CCL3, CCL4) and MMP7. Neoadjuvant therapy reactivated both subsets, evidenced by downregulation of PD-1 and increased expression of activation markers (e.g., CD38) and cytotoxic mediators (e.g., granzyme B and interferon γ). A multimodal predictive model incorporating CD8+T cell subsets (c03, c17) and plasma biomarkers (IL-5, MMP7) demonstrated superior predictive accuracy (AUC = 0.9219).
Conclusions: Integrated peripheral immune profiling enables robust, noninvasive prediction of neoadjuvant PD-1 blockade efficacy in HNSCC. The identified immune cell subsets and plasma biomarkers provide a clinically applicable framework for early response stratification and personalized immunotherapy, supporting liquid biopsy as a viable platform for clinical decision-making. Trial registration Chinese Clinical Trial Registry, clinical trial number CHiCTR2200056354, 04 February 2022, https://www.chictr.org.cn/showproj.html?proj=151364 .
Keywords: Head and neck squamous cell carcinoma; Liquid biopsy; Multi-omics research; Neoadjuvant therapy; PD-1 inhibitor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics Committee of Peking University School and Hospital of Stomatology (No. PKUSSIRB-202170179 and No. PKUSSIRB-202276072). And written informed consent was obtained from all participants. Consent for publication: All the authors approved the publication. Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- Vos JL, Elbers JBW, Krijgsman O, Traets JJH, Qiao X, van der Leun AM, Lubeck Y, Seignette IM, Smit LA, Willems SM, et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat Commun. 2021;12:7348. - PMC - PubMed
-
- Jubran MR, Vilenski D, Flashner-Abramson E, Shnaider E, Vasudevan S, Rubinstein AM, Meirovitz A, Sharon S, Polak D, Kravchenko-Balasha N. Overcoming resistance to EGFR monotherapy in HNSCC by identification and inhibition of individualized cancer processes. Theranostics. 2022;12:1204–19. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

