Comparative efficacy of remifentanil and fentanyl in mechanically ventilated ICU patients: a systematic review and meta-analysis on ventilation duration and delirium incidence
- PMID: 40544288
- PMCID: PMC12182676
- DOI: 10.1186/s44158-025-00258-7
Comparative efficacy of remifentanil and fentanyl in mechanically ventilated ICU patients: a systematic review and meta-analysis on ventilation duration and delirium incidence
Abstract
Background: The ultrashort-acting properties and organ-independent metabolism of remifentanil may be advantageous in mechanical ventilation management. Unlike fentanyl, which accumulates over time and may prolong sedation, remifentanil enables more predictable titration and rapid weaning. This study aimed to determine the effect of remifentanil on shortening the duration of mechanical ventilation in comparison with fentanyl in adult intensive care unit (ICU) patients.
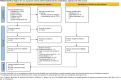
Methods: A systematic review and meta-analysis was conducted, including randomised controlled trials (RCTs) and observational studies from MEDLINE, Cochrane, EMBASE, ICTRP, and ClinicalTrials.gov, from inception to July 2024. Studies comparing remifentanil with fentanyl in mechanically ventilated ICU patients were included, whereas those that used only remifentanil or fentanyl intraoperatively were excluded. The primary outcome was ventilation duration, with a minimal important difference (MID) of 90 min. A random-effects meta-analysis was performed and the certainty of evidence was assessed using the GRADE approach. The risk of bias was evaluated using RoB 2.0 and ROBINS-I tools.
Results: We included 18 studies (14 RCTs and 4 observational studies). Ten studies (8 RCTs and 2 observational studies; 901 patients) were analysed. Remifentanil may reduce ventilation duration compared to fentanyl (8 RCTs: MD -6.70 h, 95% CI -14.36 to 0.97; low certainty; 2 observational studies: MD -21.26 h, 95% CI -37.29 to -5.24; low certainty).
Conclusions: Remifentanil may reduce the duration of mechanical ventilation, potentially improving patient outcomes. However, owing to the low certainty of the evidence and study heterogeneity, further high-quality RCTs are required to validate these findings.
Trial registration: PROSPERO 2024 and CRD42024557414.
Keywords: Critical care; Fentanyl; Mechanical ventilation; Meta-analysis; Remifentanil; Systematic review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP et al (2018) Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 46:e825–e873. 10.1097/CCM.0000000000003299 - DOI - PubMed
-
- Doi M, Takahashi N, Nojiri R, Hiraoka T, Kishimoto Y, Inoue S et al (2023) Efficacy, safety, and pharmacokinetics of MR13A11A, a generic of remifentanil, for pain management of Japanese patients in the intensive care unit: a double-blinded, fentanyl-controlled, randomized, non-inferiority phase 3 study. J Intensive Care 11:51. 10.1186/s40560-023-00698-9 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
