GDF-15 upregulates the SLC7A11/GPX4 signaling axis and promotes mitoxantrone resistance in AML cells
- PMID: 40544302
- PMCID: PMC12181867
- DOI: 10.1186/s40001-025-02787-x
GDF-15 upregulates the SLC7A11/GPX4 signaling axis and promotes mitoxantrone resistance in AML cells
Abstract
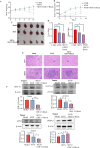
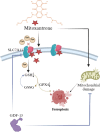
Chemotherapy resistance poses a significant challenge in the initial treatment of acute myeloid leukemia (AML). Growth differentiation factor 15 (GDF-15) has been shown to play a critical role in cancer progression; however, the potential mechanisms by which GDF-15 contributes to AML progression and chemotherapy resistance remain unclear. We found that M2 macrophages secrete high levels of GDF-15, promoting resistance of AML cells to mitoxantrone (MTX). Furthermore, we demonstrated that MTX induces downregulation of the SLC7A11/GPX4 signaling axis in AML cells, mediating ferroptosis. GDF-15 enhances the expression of the SLC7A11/GPX4 axis, thereby inhibiting ferroptosis in AML cells and contributing to drug resistance. In addition, GDF-15 mitigates the decline in mitochondrial membrane potential and mitochondrial quality induced by MTX. In vivo experiments indicate that blocking GDF-15 effectively enhances the sensitivity of AML cells to mitoxantrone by reducing the expression of the SLC7A11/GPX4 axis.
Keywords: Acute myeloid leukemia; Chemoresistance; Ferroptosis; GDF-15.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This animal study was reviewed and approved by the Experimental Animal Welfare and Ethics Committee of the Third Military Medical University on March 5, 2021 (SYXK (Ethics) 20,170,002). Primary AML cells were obtained from the bone marrow blood samples of leukemia patients with informed consent. The study protocol was approved by the Ethics Committee of Shanghai Xinchao Biotechnology Co., Ltd. and complies with the Declaration of Helsinki (Permit No.: SHXC2021YF02). Institutional review board: Not applicable. Informed consent: Not applicable. Competing interest: The authors declare no competing interests.
Figures




Similar articles
-
Dioscin induces ferroptosis to suppress the metastasis of gastric cancer through the SLC7A11/GPX4 axis.Free Radic Res. 2025 May;59(5):426-441. doi: 10.1080/10715762.2025.2515202. Epub 2025 Jun 13. Free Radic Res. 2025. PMID: 40460255
-
[Transcriptomics and Metabolomics Analysis to Explore the Ferroptosis Susceptibility of Venetoclax-Resistant AML Cells].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2025 Jun;33(3):621-632. doi: 10.19746/j.cnki.issn.1009-2137.2025.03.001. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2025. PMID: 40613148 Chinese.
-
Huangqin decoction alleviates chemotherapy-induced intestinal injury by inhibiting ferroptosis via modulation of P53/SLC7A11/GPX4 pathway.J Ethnopharmacol. 2025 Jul 24;351:120135. doi: 10.1016/j.jep.2025.120135. Epub 2025 Jun 11. J Ethnopharmacol. 2025. PMID: 40513923
-
Emerging Therapeutic Strategies Targeting GPX4-Mediated Ferroptosis in Head and Neck Cancer.Int J Mol Sci. 2025 Jul 4;26(13):6452. doi: 10.3390/ijms26136452. Int J Mol Sci. 2025. PMID: 40650229 Free PMC article. Review.
-
A systematic overview of chemotherapy effects in acute myeloid leukaemia.Acta Oncol. 2001;40(2-3):231-52. doi: 10.1080/02841860151116321. Acta Oncol. 2001. PMID: 11441935
Cited by
-
Ferroptosis: a novel pharmacological mechanism against multiple myeloma.Front Pharmacol. 2025 Jul 15;16:1606804. doi: 10.3389/fphar.2025.1606804. eCollection 2025. Front Pharmacol. 2025. PMID: 40735482 Free PMC article. Review.
References
-
- Tikhomirov AS, Shtil AA, Shchekotikhin AE. Advances in the discovery of anthraquinone-based anticancer agents. Recent Pat Anti-Cancer Drug Discovery. 2018;13(2):159–83. - PubMed
-
- Chen Z, Shi T, Zhang L, Zhu P, Deng M, Huang C, Hu T, Jiang L, Li J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: a review of the past decade. Cancer Lett. 2016;370(1):153–64. - PubMed
-
- Shahar N, Larisch S. Inhibiting the inhibitors: targeting anti-apoptotic proteins in cancer and therapy resistance. Drug Resist Updates. 2020;52: 100712. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

