Decoding the impact of exercise and αCGRP signaling on murine post-traumatic osteoarthritis progression
- PMID: 40544314
- PMCID: PMC12181913
- DOI: 10.1186/s13075-025-03589-6
Decoding the impact of exercise and αCGRP signaling on murine post-traumatic osteoarthritis progression
Abstract
Background: Osteoarthritis (OA) is a chronic degenerative joint disease characterized by cartilage breakdown, subchondral bone remodeling, and inflammation. Mechanical stress, such as exercise, can influence OA progression, acting as either a therapeutic intervention or a risk factor depending on intensity. The sensory neuropeptide αCGRP plays a role in modulating cartilage, bone, and inflammatory responses, making it a potential mediator of exercise effects on OA. This study investigated the impact of αCGRP deficiency and exercise intensity on OA progression in a post-traumatic murine model.
Methods: OA was induced in male αCGRP knockout (KO) and wild type (C57Bl/6J) mice via destabilization of the medial meniscus (DMM). Mice underwent moderate or intense treadmill exercise for up to 6 weeks (8 weeks post-surgery). Histological analyses were performed to assess cartilage degradation. Subchondral and metaphyseal bone morphology as well as cartilage stiffness were evaluated by nanoCT and atomic force microscopy (AFM), respectively. Serum inflammatory markers were analyzed using multiplex immunoassays.
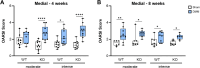
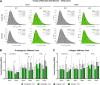
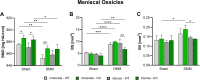
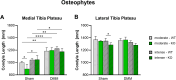
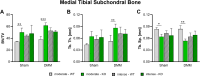
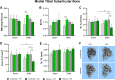
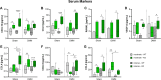
Results: Serum levels of proinflammatory markers were elevated in αCGRP-deficient mice, particularly after intense exercise, independent of OA progression. DMM surgery induced significant cartilage degradation. Gross cartilage morphology was not influenced by exercise intensity or αCGRP deficiency, but αCGRP deficiency prevented articular cartilage extracellular matrix stiffening after DMM and intense exercise. Subchondral bone sclerosis was induced by αCGRP deficiency and DMM but mitigated by intense exercise. In metaphyseal bone, intense exercise induced trabecular loss in αCGRP-deficient mice.
Conclusions: This study highlights αCGRP as an intrinsic regulator of joint and bone responses to mechanical loading during OA. While cartilage degradation after DMM and treadmill exercise was unaffected by lack of αCGRP, its deficiency altered ECM stiffness, bone remodeling, and inflammatory responses. These findings position αCGRP as a critical regulator of joint homeostasis, particularly for bone health during running exercise and OA progression.
Keywords: Alpha-calcitonin gene-related peptide; Bone; Cartilage; Destabilization of the medial meniscus; Exercise; Osteoarthritis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Title of the project: Untersuchung zum Einfluss des sensiblen Nervensystems auf Veränderungen des osteoarthrotischen subchondralen Knochengewebes unter definierter mechanischer Belastung (Laufbandtraining). The local authorities in Würzburg (Regierung von Unterfranken) approved all animal experiments. Approval number: AZ 55.2-2532-2-1253. Date of approval: November 5, 2020. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Effects of intra-articular applied rat BMSCs expressing alpha-calcitonin gene-related peptide or substance P on osteoarthritis pathogenesis in a murine surgical osteoarthritis model.Stem Cell Res Ther. 2025 Mar 5;16(1):117. doi: 10.1186/s13287-025-04155-2. Stem Cell Res Ther. 2025. PMID: 40045368 Free PMC article.
-
Receptor activator of nuclear factor-kappa B ligand-derived microglia healing peptide 1-AcN inhibits osteoarthritis progression in mice.Arthritis Res Ther. 2025 Jul 9;27(1):142. doi: 10.1186/s13075-025-03609-5. Arthritis Res Ther. 2025. PMID: 40635000 Free PMC article.
-
Chondrocyte-specific knockout of Piezo1 and Piezo2 protects against post-traumatic osteoarthritis structural damage and pain in mice.Arthritis Res Ther. 2025 Jul 19;27(1):152. doi: 10.1186/s13075-025-03620-w. Arthritis Res Ther. 2025. PMID: 40684207 Free PMC article.
-
Exercise for hand osteoarthritis.Cochrane Database Syst Rev. 2017 Jan 31;1(1):CD010388. doi: 10.1002/14651858.CD010388.pub2. Cochrane Database Syst Rev. 2017. PMID: 28141914 Free PMC article.
-
Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review.Cochrane Database Syst Rev. 2018 Apr 17;4(4):CD010842. doi: 10.1002/14651858.CD010842.pub2. Cochrane Database Syst Rev. 2018. PMID: 29664187 Free PMC article.
References
-
- Muschter D, Fleischhauer L, Taheri S, Schilling AF, Clausen-Schaumann H, Grässel S. Sensory neuropeptides are required for bone and cartilage homeostasis in a murine destabilization-induced osteoarthritis model. Bone. 2020;133:115181. - PubMed
-
- Raposo F, Ramos M, Lúcia Cruz A. Effects of exercise on knee osteoarthritis: A systematic review. Musculoskelet Care. 2021;19:399–435. - PubMed
-
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–30. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

