Sequencing of chemotherapy in total neoadjuvant treatment for rectal cancer does not predict radiation-induced lymphopenia
- PMID: 40544503
- PMCID: PMC12182946
- DOI: 10.2478/raon-2025-0034
Sequencing of chemotherapy in total neoadjuvant treatment for rectal cancer does not predict radiation-induced lymphopenia
Abstract
Background: Radiation-induced lymphopenia (RIL) is associated with an increased risk of death in solid tumors, including rectal cancer. The aim of this study was to determine whether the sequencing of chemotherapy in total neoadjuvant treatment (TNT) for rectal cancer predicts the development of RIL.
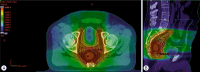
Patients and methods: We analyzed acute hematologic toxicity data from 53 patients who underwent TNT for locally or locoregionally advanced rectal cancer between July 2022 and April 2023. Twenty-eight patients received induction chemotherapy with capecitabine and oxaliplatin [CAPOX], and 25 received consolidation chemotherapy (6 cycles of CAPOX in both groups). The chemoradiation protocol consisted of Volumetric Modulated Arc Therapy with Simultaneous Integrated Boost Radiotherapy (VMAT-SIB RT) up to 48.4 Gy in 22 fractions, concomitantly with capecitabine twice a day (lat. bis in die, BID). The Mann-Whitney U test was performed to compare RIL between the two patient groups. Pelvic bone marrow was contoured as a non-limiting organ-at-risk to assess the received dose, and binary logistic regression was used to determine whether RIL depends on V5Gy~V42Gy or the planning target volume (PTV) size.
Results: Thirty-four patients (64.2%) developed RIL of any grade, which was not significantly associated with either the induction or consolidation chemotherapy TNT regimen (Wald = 3.159, p = 0.076). No significant differences were found in neutrophil counts or the neutrophil-to-lymphocyte ratio. In the logistic regression model predicting the likelihood of RIL, two variables were statistically significant: V10Gy (Wald = 4.366, p = 0.037) and V30Gy (Wald = 6.084, p = 0.014). These results indicate that V10Gy< 71% and V30Gy< 26.6% may reduce the likelihood of developing RIL.
Conclusions: In our study, the sequencing of chemotherapy in TNT for rectal cancer did not predict the development of RIL. However, the incidence of RIL may be reduced by applying RT dosimetric constraints to the pelvic bone marrow.
Keywords: radiation-induced lymphopenia; rectal cancer; total neoadjuvant treatment.
© 2025 Miha Orazem et al., published by Sciendo.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous