Caged-hypocrellin mediated photodynamic therapy induces chromatin remodeling and disrupts mitochondrial energy metabolism in multidrug-resistant Candida auris
- PMID: 40544603
- PMCID: PMC12221887
- DOI: 10.1016/j.redox.2025.103708
Caged-hypocrellin mediated photodynamic therapy induces chromatin remodeling and disrupts mitochondrial energy metabolism in multidrug-resistant Candida auris
Abstract
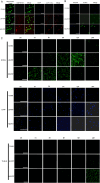
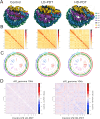
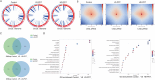
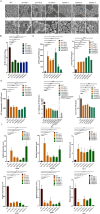
Candida auris is a fungal pathogen with frequent development of multidrug-resistance or pan-drug resistance. Currently, the treatment options for Candida auris are limited. Therefore, there is an urgent need for alternative therapeutic strategies. Antimicrobial photodynamic therapy (aPDT), which generates reactive oxygen species (ROS) through light-activated photosensitizers, has shown promise against C. auris; however, its molecular mechanism remains unclear. To investigate COP1T-HA-mediated PDT-induced genomic alterations, we constructed a 3D genome map of Candida species, which uncovered the reorganization of chromatin architecture in response to PDT treatment. Our data showed that low-dose PDT causes subtle local adjustments in chromatin topology, whereas high-dose PDT leads to more pronounced changes in A/B compartmentalization, topologically associating domain (TAD) organization, and chromatin looping associated with key genes related to mitochondrial energy metabolism. Confocal imaging confirmed that high-dose COP1T-HA-mediated PDT induces localized ROS accumulation near the nucleus and a temporally ordered cellular stress response. Furthermore, functional validation through QCR10, NDUFA5, and MP knockouts confirmed the essential roles of these genes in mitochondrial integrity, ATP synthesis, ROS homeostasis, and biofilm formation. Mutants showed altered mitochondrial membrane potential, intracellular pH imbalance, and enhanced glycolytic compensation, highlighting the impact of electron transport disruption on energy metabolism. This study provides the first comprehensive insight into COP1T-HA-mediated PDT-induced chromatin reorganization in C. auris and establishes a direct connection between 3D genome remodeling and fungal energy metabolism, offering a foundation for chromatin-targeted antifungal strategies.
Keywords: 3D genome architecture; Chromatin remodeling; Mitochondrial energy metabolism; Multidrug-resistant Candida auris; Photodynamic therapy (PDT).
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Denning D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024;24:e428–e438. - PubMed
-
- Meis J.F., Chowdhary A. Candida auris: a global fungal public health threat. Lancet Infect. Dis. 2018;18:1298–1299. - PubMed
-
- Rhodes J., Fisher M.C. Global epidemiology of emerging Candida auris. Curr. Opin. Microbiol. 2019;52:84–89. - PubMed
-
- Satoh K., Makimura K., Hasumi Y., Nishiyama Y., Uchida K., Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009;53:41–44. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

